Parent’s Guide to Cord Blood, June 2025
Back Story of Cell Therapy for Pediatric Neurodevelopment Disorders
Since 2005, cells from cord blood or umbilical cord tissue have been studied in numerous clinical trials aiming to treat newborn brain injuries and neurodevelopmental disorders[1]. Many parents were persuaded to sign contracts to store their child’s cord blood and tissue based on promises from these studies. However, the journey from clinical trials to widely available therapies has been slow and frustrating. There has been pushback from consumers that cord blood banking has not lived up to its promise[2].
The pediatric developmental disorders studied using cells from cord blood or cord tissue have very diverse symptoms. Clinical trials have not followed a standardized approach in describing patients or therapy procedures, making it difficult to compare study results.
Moreover, many studies have a “cross-over” design, meaning that the control group also receives the treatment after a delay. If the delay is too short, it becomes hard to detect treatment effects[3]. Some studies fail to meet their endpoints because only a subset of participants experience clear benefits from the therapy[4,5]. Seeking funding for this research also contributes to delays[6].
New Evidence of Cell Therapy Efficacy for Cerebral Palsy
In spring 2025, two important papers were published, proving the effectiveness of cell therapy for cerebral palsy. Both were meta-analyses of previous studies. Their results are a big boost for the field of perinatal cell therapy.
Why this matters:
- These studies provide the first conclusive evidence that cell therapy can benefit pediatric neurodevelopmental disorders.
- Both focus on cerebral palsy—the most common motor disorder in children[7], affecting 10–15% of babies born prematurely[8].
- April 2025: The journal Pediatrics published a study showing that cord blood therapy significantly improves motor skills in children with cerebral palsy[9,10].
- May 2025: The journal Cells published a study showing that mesenchymal stromal cells (MSC), mostly from cord tissue, also improve motor skills in the same group of children[11].
Comparing Statistics of Cord Blood versus MSC for Cerebral Palsy
Note: The two studies used different analysis methods, so their results are not directly comparable.
| Criteria | Cord Blood (IPDMA) | MSC (Meta-analysis) |
| Type of analysis | Individual Participant Data Meta-Analysis (stronger) | Aggregate Meta-Analysis |
| Number of children | 447 total (170 treated, 171 controls at 12 months) | 1292 total (138 treated, 143 controls at 12 months) |
| 12-month benefit | Increase of 1.42 GMFM points (p=0.012) – large effect | Increase of 0.99 GMFM points (p=0.005) – large effect |
| Data quality | Mostly from published clinical trials | Mostly from compassionate access programs |
| Cell source | 84% from donated cord blood | 71–80% from donated cord tissue |
| Dosing | Single dose | 72% received multiple doses |
| MSC type | – | 75% from umbilical cord tissue (UC-MSC) |
| Subgroup response | Better in children <5 years, mild CP (GMFCS 1–3), higher doses (p=0.047) | No specific best responders identified, but multiple doses had nearly double benefit (0.65 → 1.24 GMFM) |
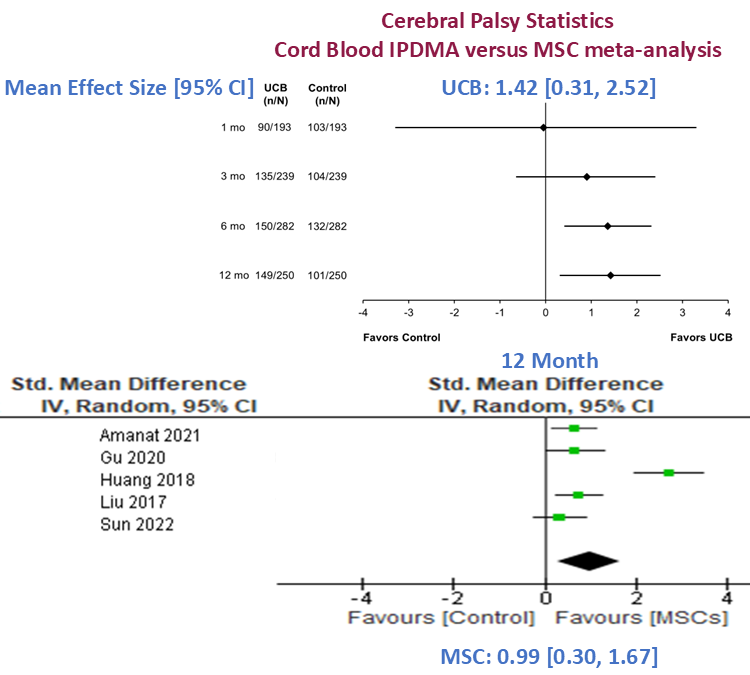
Image combines forest plots from Fig.1 of Finch-Edmondson et al. 2025 [10] & Fig.4 of Paton et al. 2025 [11].
What’s Next for People Living With Cerebral Palsy?
Most countries have not yet approved cell therapy as an official treatment for cerebral palsy without a successful phase 3 trial. Full market approval is typically required for insurance coverage and broad implementation at medical centers.
However, some countries offer the therapy through compassionate access programs:
- USA: Since 2017, Dr. Joanne Kurtzberg’s team at Duke University has offered cord blood therapy for cerebral palsy through an “Expanded Access” program. Families must pay for treatment themselves[12].
- Europe: The FamiCord Group – Europe’s largest cord blood bank network – partners with University Children’s Hospital in Lublin, Poland, to provide free therapy for children who banked their cord blood at FamiCord and pre-registered for treatment[13].
- Australia: In April 2025, the first case was approved under the Special Access Scheme (SAS) – Category B to receive their own cord blood. Unlike the US or EU programs, local doctors can apply under SAS, and the national healthcare system covers the cost if approved[14].
When we look at the promise of cord blood and cord tissue as a form of regenerative medicine, that promise is becoming more of a reality for families living with cerebral palsy.
References
- Sun J, Allison J, McLaughlin C, Sledge L, Waters-Pick B, Wease S, et al. Differences in quality between privately and publicly banked umbilical cord blood units: a pilot study of autologous cord blood infusion in children with acquired neurologic disorders. Transfusion. 2010; 50(9):1980–7.
- Wade G. Cord blood banking is not living up to its promise. New Scientist. Published 2025-05-26. (This article speaks only about cord blood for transplants, apparently the journalist was not aware of the latest research results for cerebral palsy.)
- Chez M, Lepage C, Parise C, Dang‐Chu A, Hankins A, Carroll M. Safety and Observations from a Placebo‐Controlled, Crossover Study to Assess Use of Autologous Umbilical Cord Blood Stem Cells to Improve Symptoms in Children with Autism. SCTM 2018; 7(4):333–341.
- Kurtzberg J. Results from the Duke ACT Study of Cord Blood for Autism: The Inside Scoop from Dr. Kurtzberg. Parent’s Guide to Cord Blood Foundation Newsletter Published 2020-07.
- Dawson G, Sun JM, Baker J, Carpenter K, Compton S, Deaver M, … Kurtzberg J. A Phase II Randomized Clinical Trial of the Safety and Efficacy of Intravenous Umbilical Cord Blood Infusion for Treatment of Children with Autism Spectrum Disorder. Journal of Pediatrics. 2020; 222:164–173.
- Paton MCB, Finch-Edmondson M, Fahey MC, London J, Badawi N, Novak I. Fifteen years of human research using stem cells for cerebral palsy: A review of the research landscape. J. Paediatrics Child Health. 2021; 57(2):295–296.
- Centers for Disease Control. About Cerebral Palsy. Last updated 2025-03-17.
- Stavsky M, Mor O, Mastrolia SA, Greenbaum S, Than G, Erez O. Cerebral Palsy—Trends in Epidemiology and Recent Development in Prenatal Mechanisms of Disease, Treatment, and Prevention. Frontiers Pediatrics. 2017; 5:21.
- Verter F. Cord Blood Proven Effective for Cerebral Palsy. Parent’s Guide to Cord Blood Foundation Newsletter Published 2025-04.
- Finch-Edmondson M, Paton M, Webb A, Ashrafi M, Blatch-Williams R, Cox CJ, … Novak I. Cord Blood Treatment for Children With Cerebral Palsy: Individual Participant Data Meta-Analysis. Pediatrics. 2025; 155(5):e2024068999.
- Paton MCB, Griffin AR, Blatch-Williams R, Webb A, Verter F, Silva Couto P, … Finch-Edmondson M. Clinical Evidence of Mesenchymal Stromal Cells for Cerebral Palsy: Scoping Review with Meta-Analysis of Efficacy in Gross Motor Outcomes. Cells. 2025; 14(10):700.
- Verter F. Parent’s Guide to Duke’s Research and Expanded Access Program of Cord Blood Therapies for Neurodevelopmental Disorders. Parent’s Guide to Cord Blood Foundation Newsletter Published 2021-11.
- Verter F. Cerebral Palsy Expanded Access Program in Europe. Parent’s Guide to Cord Blood Foundation Newsletter Published 2019-11.
- Cerebral Palsy Alliance. Umbilical cord blood and cerebral palsy. Advocacy. Published 2025-05-22.
Source: Parent’s Guide to Cord Blood
Link: https://parentsguidecordblood.org/en/news/cord-blood-banking-starts-live-its-promise








