BMJ,05/06/2025
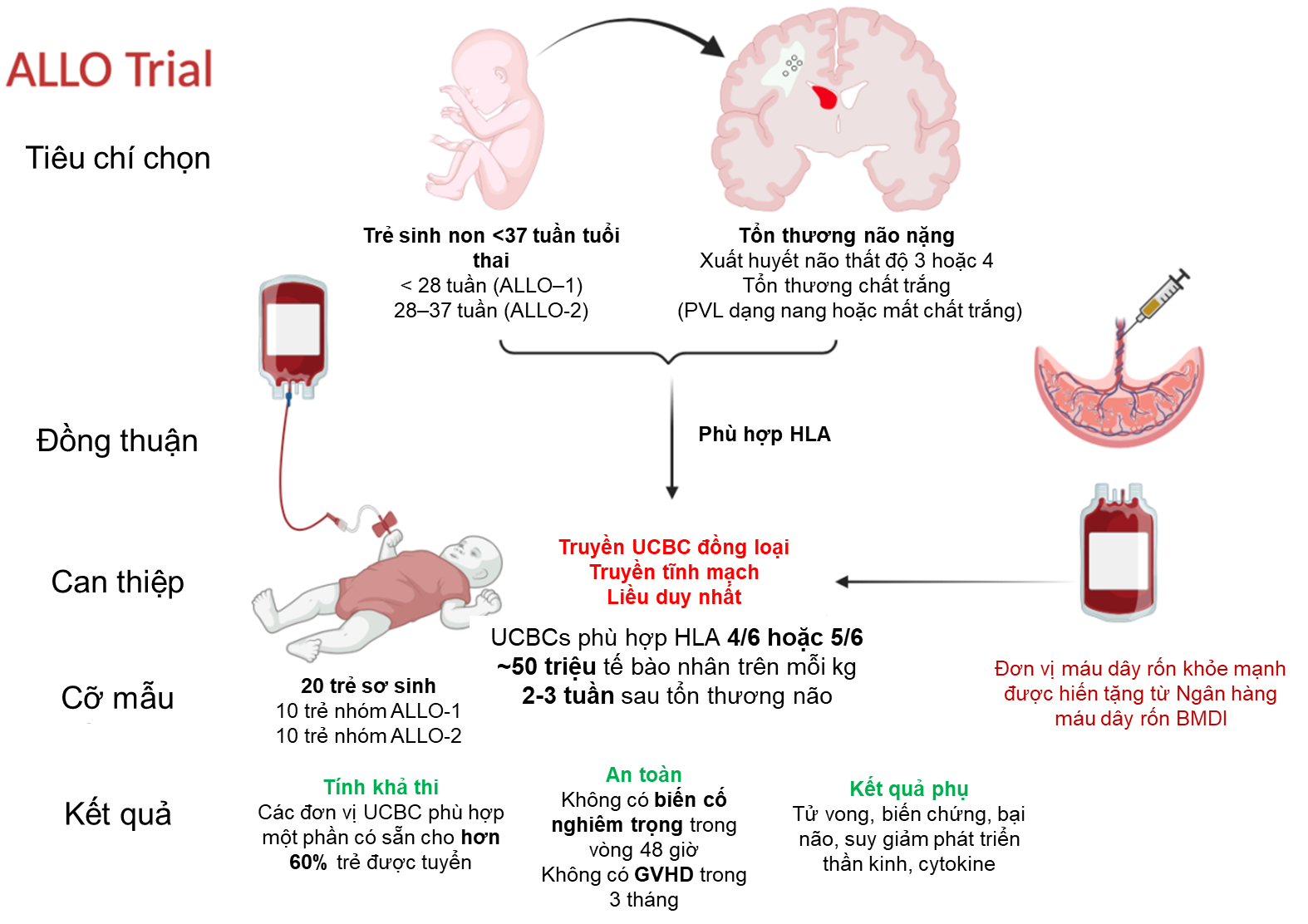
A pioneering clinical trial – the ALLO Trial – is currently being conducted at Monash Children’s Hospital to evaluate the safety and feasibility of allogeneic cord blood-derived cell therapy in treating severe brain injury in preterm infants. The results of this study are expected to pave the way for new neuroregenerative treatment strategies that could improve outcomes for infants at high risk of developing cerebral palsy.
Background
Early brain injury in preterm infants – such as grade 3–4 intraventricular hemorrhage (IVH) and white matter injury (WMI) – is one of the leading causes of cerebral palsy and long-term neurodevelopmental impairment. Although advances in neonatal care have improved survival, there is currently no effective treatment for these long-term neurological sequelae.
A promising solution: Umbilical Cord Blood Cells (UCBCs)
Umbilical cord blood cells have demonstrated anti-inflammatory and neuroregenerative potential in preclinical studies. However, the use of autologous cord blood is often not feasible in preterm infants, as early delivery usually precludes timely collection. Therefore, allogeneic therapy using donor-derived cord blood is emerging as a promising alternative.
About the ALLO Trial
- Study type: Phase I, single-arm, non-randomized, open-label clinical trial.
- Study participants:
- Infants born before 28 weeks gestation (ALLO-1) or between 28 and 36+6 weeks (ALLO-2).
- Diagnosed with severe brain injury based on ultrasound or MRI.
- Intervention:
- Single intravenous infusion of partially HLA-matched (4/6 or 5/6) allogeneic UCBCs.
- Dose: 50 million total nucleated cells (TNC) per kg of body weight.
- Cord blood units are sourced from the BMDI Cord Blood Bank, part of the AusCord network, ensuring national quality standards.
Study Objectives
- Feasibility: To achieve a ≥60% match rate for eligible participants with a suitable cord blood unit.
- Safety: Defined as the absence of serious adverse events within 48 hours post-infusion, and no graft-versus-host disease (GVHD) within 3 months.
Follow-up and Assessment
Infants will be monitored until 24–36 months corrected age. Outcomes include:
- Survival rate
- Incidence of cerebral palsy, developmental delay, vision or hearing impairment
- Neurodevelopmental assessments using Bayley-4, HINE, and GM scales
- Inflammatory cytokine profiling before and after infusion to explore response mechanisms
Timeline and Milestones
- Recruitment period: From May 2024 until 20 infants (10 per group) are enrolled.
- Expected study completion: End of 2027
- Interim safety analysis: After the first five participants in each group receive the intervention.
Significance of the Study
The ALLO Trial is the first study worldwide to investigate allogeneic UCBC therapy in preterm infants with brain injury. Demonstrating feasibility and safety will lay the groundwork for larger-scale trials to evaluate the true efficacy of cord blood cell therapy in improving neurodevelopmental outcomes.
References
The article was translated and summarized from the research paper: Razak, A., Connelly, K., Hunt, R. W., et al. (2025). Safety and feasibility of allogeneic cord blood-derived cell therapy in preterm infants with severe brain injury (ALLO trial): A phase-1 trial protocol. BMJ Open, 15, e100389. https://doi.org/10.1136/bmjopen-2025-100389
Source: BMJ
Link: https://bmjopen.bmj.com/content/15/6/e100389








