Cell Biochemistry and Biophysics, 01 August 2025
Introduction
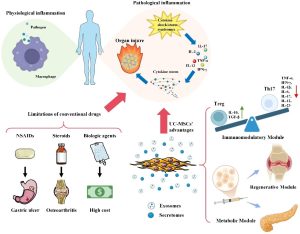
Psoriasis is a chronic inflammatory skin disease with a pathogenesis closely related to immune dysregulation. Its hallmark lesions are red, thickened skin plaques covered with silvery-white scales, sometimes accompanied by itching or burning. Approximately 20–30% of patients also develop psoriatic arthritis, causing pain and limited mobility.
Currently, there is no definitive cure. Treatment regimens mainly aim to reduce symptoms and control flare-ups through topical corticosteroids, vitamin D analogs, phototherapy, or systemic agents (methotrexate, cyclosporine, biologics targeting TNF-α, IL-17, IL-23). However, these methods still have limitations: adverse effects, diminished efficacy over time, and inability to address the underlying causes.
Mesenchymal Stem Cells (MSCs) – A Novel Therapeutic Candidate
MSCs are adult stem cells capable of self-renewal and differentiation into various cell types. They can be derived from diverse sources:
- Bone marrow
- Adipose tissue
- Umbilical cord blood
- Wharton’s jelly of the umbilical cord
- Dental pulp
The distinctive feature that makes MSCs attractive for psoriasis treatment is their potent immunomodulatory capacity:
- Secretion of anti-inflammatory cytokines (IL-10, TGF-β)
- Suppression of pro-inflammatory immune cells (Th17, NK cells, dendritic cells)
- Activation of regulatory T cells (Tregs) to restore immune balance
- Support for tissue regeneration and restoration of skin architecture
Potential Mechanisms of Action in Psoriasis
- Suppression of inflammatory responses: reducing IL-17 and IL-22 production, key cytokines in skin lesions
- Increase in Tregs: restoring immune homeostasis
- Reduction of keratinocyte hyperproliferation: preventing excessive scaling and epidermal thickening
- Stimulation of tissue repair: via growth factor secretion and angiogenesis
Notable Research Findings
- Preclinical Studies
Imiquimod (IMQ)-induced psoriasis mouse model
- Intervention: Subcutaneous injection of human umbilical cord MSCs (hUC-MSCs)
- Results:
- Reduced epidermal hyperkeratosis and inflammatory infiltration
- Decreased activation of IL-17–producing γδ T cells
- Increased Tregs, improving immune balance
MSC-derived exosomes
- Characteristics: Extracellular vesicles (15–30 nm) containing regulatory proteins and RNA
- Effects:
- Activation of TGF-β2 signaling → inhibition of keratinocyte proliferation
- Reduced skin inflammation, restoration of epidermal structure
MSC secretome combined with hyaluronic acid (HA)
- Formulation: Topical hydrogel/foam
- Animal study outcomes:
- Safe, non-irritating
- Enhanced angiogenesis and skin healing
- Marked reduction in erythema, scaling, and inflammation
- Early Clinical Studies
Intravenous or local MSC administration
- Cell source: Fresh or cryopreserved UC-MSCs
- Results:
- Significant reduction in PASI scores after 12 weeks
- Improvement in both physician (PGA) and patient (PtGA) assessments
- No serious treatment-related adverse events
Different MSC sources
- Bone marrow, adipose tissue, and umbilical cord showed comparable anti-inflammatory and lesion-reducing effects
- Some cases maintained improvement for over 6 months after a single treatment course
Topical exosome therapy
- Formulation: MSC-derived exosome cream
- Effects:
- Itching and redness reduced within 2–4 weeks
- Near-normal skin architecture under histological examination
- Scientific Implications
- MSCs provide dual benefits: anti-inflammatory action and tissue regeneration—an advantage over most current therapies
- Acellular products such as exosomes and secretomes may offer greater safety, easier storage, and standardization potential
Challenges and Prospects
Challenges
- MSCs derived from psoriasis patients may have impaired function
- Lack of consensus on optimal cell source, dosage, and administration route
- Risk of vascular occlusion with intravenous injection if protocols are not standardized
Prospects
- Shift towards cell-free therapies (exosomes/secretomes)
- GMP-compliant manufacturing standardization
- Large-scale, multicenter clinical trials to confirm efficacy and safety
Conclusion
Mesenchymal stem cells and their derivatives are emerging as a novel approach for psoriasis treatment, with the potential to combine immune regulation and skin regeneration. However, widespread application will require overcoming challenges in standardization, safety assessment, and protocol optimization.
References
The article was summarized from the research paper: Wong, R. S. Y., Chua, K. H., Tan, E. W., & Goh, B. H. (2025). Therapeutic Potential of Mesenchymal Stem Cells in Psoriasis. Cell Biochemistry and Biophysics, 1-15.
Source: Cell Biochemistry and Biophysics
Link: https://link.springer.com/article/10.1007/s12013-025-01843-x#citeas