Stem Cell Research & Therapy, 13/10/2025
Background
One of the deadliest chronic illnesses in the world is diabetes. It’s predicted global prevalence was 10.5% in 2019 (536.6 million people), and by 2045, it is expected to rise to 12.2% (783.2 million people). Diabetic foot ulcers (DFUs) are a significant complication associated with type 2 diabetes mellitus (T2DM), which cause 85% of diabetic lower-limb amputations (DLLA), are one of its worst side effects. Significant mortality and high recurrence rates are other characteristics of DFU; more than 40% of afflicted individuals pass away within 5 year. Diabetic foot ulcers (DFUs) are among the most severe chronic complications of diabetes, significantly impairing patients’ quality of life and contributing to high rates of disability and amputation.
Current treatment strategies focus on improving blood flow, controlling infection with antibiotics, and utilizing specialized wound-healing dressings. However, the effectiveness of these approaches is often limited, particularly in advanced cases, as they fail to address the underlying biological mechanisms that delay healing.
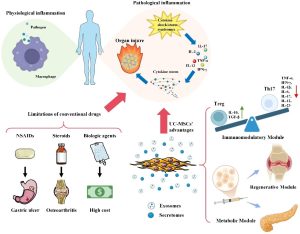
In recent years, researchers have begun to explore novel techniques involving stem cells and extracellular vesicles (exosomes) as innovative solutions for treating chronic wounds. Exosomes generated from mesenchymal stem cells (MSCs) have attracted interest in regenerative medicine in recent years. Exosomes are tiny extracellular vesicles that carry proteins, lipids, and nucleic acids, therefore promoting intercellular communication. Exosomes generated from MSCs have shown promise in reducing inflammation, fostering angiogenesis, and improving tissue healing in several ailments, such as chronic wounds, osteoarthritis, and cardiovascular disorders. Wharton’s jelly mesenchymal stem cells (WJ-MSCs), which are formed from Wharton’s jelly (WJ), are distinct from other sources of umbilical cord-derived MSCs. WJ-MSCs have several benefits, including a high potential for differentiation and immune purification, and are very accessible and morally uncontroversial. Additionally, they have traits with embryonic stem cells, such as strong expansion potential and rapid cell division.
MSC-exosomes have demonstrated a potent anti-inflammatory function; they may enhance M2 polarization over M1 by regulating inflammatory cytokine levels, which includes downregulating pro-inflammatory mediators like TNF-α and IL-1β and upregulating the anti-inflammatory cytokine IL-10. Additionally, by upregulating the expression of Foxp3 (a crucial transcription factor for the formation and function of regulatory T cells) and IDO, exosomes aid in T cell differentiation and encourage the transition toward anti-inflammatory phenotypes, such as regulatory T (Treg) cells. Regarding vascularization, exosome-mediated delivery of HGF (Hepatocyte growth factor) supports vascular stability and enhances neovascularization by activating the PTEN/PI3K/Akt and MAPK signaling pathways. Human umbilical cord mesenchymal stem cell-derived exosomes (hUCMSC-Exos) are enriched in miR-21, miR-23a, miR-125b, and miR-145, a specific group of miRNAs that inhibit myofibroblast activation and attenuate actin production and collagen deposition results in reduced scar formation and improved tissue remodeling in late stages of wound healing.
To explore a potential new therapeutic paradigm for this crippling illness, we set out to determine the safety and effectiveness of topical administration of WJ-MSC-derived exosomes in patients with chronic DFUs. In addition to increasing healing rates, the research’s findings may greatly raise the standard of living for those with diabetic foot ulcers.
Isolation and characterization of WJ-MSC cells from UC
After gaining informed consent, the university hospital used aseptic surgery to remove the umbilical cord tissue from a healthy donor. WJ-MSC cells’ morphology was examined under a microscope. In WJ-MSC cells during the 21st-day passage, flow cytometric examination revealed the presence of CD14, CD34, CD73, and CD105 labeling. WJ-MSC cell surface receptors CD14, CD34, CD73, and CD105 staining were subjected to the immuno fluorescence technique.
Isolation and characterization of exosomes from WJ-MSC
For 48 h, MSC cells were cultured in 75 cm2 flasks using DMEM/F12 mix that was devoid of FBS (starved). Exosomes secreted from fasting cells during a 48-h period were first obtained by collecting the media. To separate the cells and big vesicles, the fluids were centrifuged. Using flow cytometry, the characteristics of the isolated exosomes were examined for the CD9, CD63, CD81, and HSP70 markers. TEM electron microscopy was used to determine the morphology and nano-size of WJMSCs exosomes. The isolation and characterization process were repeated three times to confirm reproducibility and consistency in results.
Study design
To assess the effectiveness and safety of WJ-MSC-derived exosome gel in patients with diabetic foot ulcers (DFUs), a randomized double-blind controlled clinical experiment was carried out. The trial protocol was implemented after obtaining the Scientific Research Ethics Committee of Kafr Elsheikh University on 25/3/2024.
110 of them satisfied the requirements for inclusion. The patients were then split into three groups:
- Group treated: 40 patients received standard of care (SOC) once weekly for 4 weeks, followed by a 16-week follow-up, using Wharton jelly derived mesenchymal stem cell (WJ-MSC) exosome gel.
- Control group: 35 patients had just standard of care (SOC) for 4 weeks, then 16 weeks of follow-up.
- A visually similar saline-based formulation was administered once weekly to 35 patients in the placebo group for 4 weeks, followed by follow-up for 16 weeks, along with SOC.
All patients in the three groups mentioned above were subjected to
- To get rid of necrotic or diseased tissues, wounds were debrided.
- After cleaning the region with regular saline, the therapy was administered.
- The ulcers were covered with non-compressive bandages and sterile gauze.
- Participants were instructed to clean and redress ulcers daily.
Participants were followed up for 16 weeks
Two systems were used for classification: the Wound, Ischemia, and Foot Infection system (WIFI system) and the Site, Ischemia, Neuropathy, Bacterial Infection, Area, Depth system (SINBAD system). To classify foot infection, we use the international working group on the diabetic foot (IWGDF)/Infectious diseases society of America (IDSA system).
Results
When compared to baseline at 2 and 4 weeks, the treatment group’s ulcer area and the control group’s ulcer size were significantly reduced. Patients in both groups showed a substantial decrease in ulcer size at 6 weeks after therapy when compared to baseline, however, patients in the placebo group showed no discernible decrease in ulcer size. Patients in the treated group showed a substantial decrease in ulcer size at 2 and 4 months after therapy, as compared to baseline. In a similar vein, controls showed a notable decrease in ulcer size 2 and 4 months after therapy.
The mean time for complete healing was 6 weeks (range: 4-8 week) for the treated group and 20 weeks (range: 12-28 week) for the controls. At the end of the study, 53 patients (62%) had achieved complete healing, with the percentage of patients in the treated group achieving complete healing being significantly higher than that in the control group.
Commonly reported adverse events included infection reported in 2 cases (6%), fever 2 (6%), and blisters 3 (10%).
Conclusion
Our research adds to the increasing amount of data indicating that MSC-Exos is a viable treatment option for DFUs. MSC-Exos provide a multimodal approach to improve wound healing outcomes in diabetes patients by focusing on several pathogenic pathways, such as inflammation, angiogenesis, neuroprotection, infection control, and extracellular matrix remodeling. To maximize their therapeutic effectiveness and provide standardized methods for their clinical application, more extensive clinical trials are necessary.
References
Kishta, M.S., Hafez, A.M., Hydara, T. et al. (2025). The transforming role of wharton’s jelly mesenchymal stem cell-derived exosomes for diabetic foot ulcer healing: a randomized controlled clinical trial. Stem Cell Res Ther 16, 559. https://doi.org/10.1186/s13287-025-04690-y
Source: Stem Cell Research & Therapy
Link: https://stemcellres.biomedcentral.com/articles/10.1186/s13287-025-04690-y#citeas