Journal of Translational Medicine, 11/06/2025
Introduction
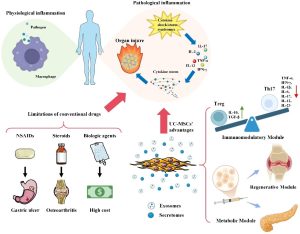
Knee osteoarthritis (OA) is the most common chronic joint disease, causing damage to cartilage, subchondral bone, and the synovial membrane. OA leads to pain, limited mobility, and is the leading cause of disability among middle-aged and elderly individuals. According to global statistics, more than 27 million Americans suffer from OA, with annual healthcare costs exceeding 180 billion USD. Currently, the main treatment approaches (pain relief, anti-inflammatory drugs, intra-articular injections, or joint replacement) only alleviate symptoms but cannot restore damaged cartilage tissue.
Over the past decade, mesenchymal stem cell (MSC) therapy has opened a new direction in the treatment of OA. MSCs possess abilities for differentiation, anti-inflammation, immune regulation, and tissue regeneration. However, the direct use of MSCs still faces limitations such as difficulties in controlling cell survival, risks of tumor formation, or immune reactions. Recent evidence suggests that exosomes—small extracellular vesicles secreted by MSCs—are the main mediators of MSC therapeutic effects. Exosomes are nanosized (50–150 nm) vesicles containing proteins, RNA, microRNA, and lipids that regulate inflammation and tissue regeneration. Compared with stem cells, exosomes are more stable, less immunogenic, and easier to store and transport.
In particular, exosomes derived from human umbilical cord mesenchymal stem cells (hUC-MSC-Exos) are considered the most effective and safest source, as the umbilical cord is a postnatal waste tissue with minimal ethical concerns and contains cells with strong proliferative capacity. Preclinical studies have demonstrated that hUC-MSC-Exos can:
- Reduce the secretion of inflammatory factors such as IL-6, TNF-α, and MMP13;
- Increase the expression of type II collagen and aggrecan in chondrocytes;
- Inhibit the NF-κB, AKT, and MAPK signaling pathways;
- Promote cartilage regeneration and reduce subchondral bone damage.
However, there is still limited clinical evidence confirming the safety and efficacy of exosome injection in humans. Therefore, the authors conducted this study to evaluate the effects of hUC-MSC-Exos from preclinical experiments to clinical trials, in order to determine their therapeutic potential for OA.
Methods
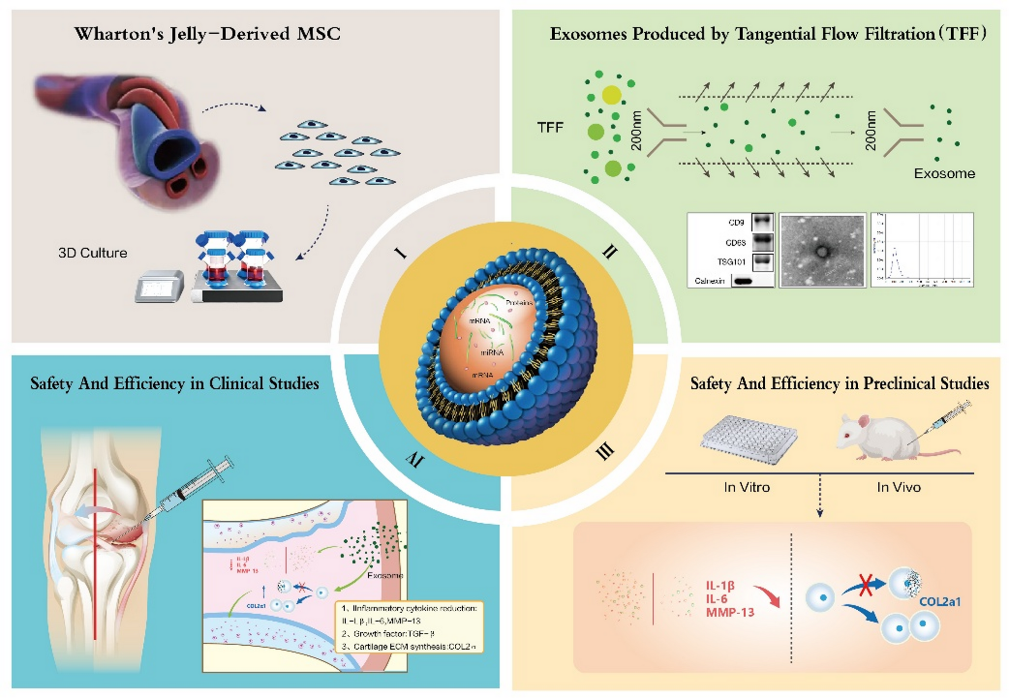
Source and characteristics of human umbilical cord mesenchymal stem cells (hUC-MSCs)
Mesenchymal stem cells were isolated from the Wharton’s jelly portion of umbilical cords obtained from healthy donors after birth, following protocols approved by the medical ethics committee. Umbilical cord samples were washed with PBS containing penicillin/streptomycin to remove blood and debris. The tissue was then finely minced, digested with collagenase type I and trypsin, and subsequently cultured in DMEM/F12 medium supplemented with 10% fetal bovine serum (FBS). The cells were maintained at 37 °C in a 5% CO₂ incubator, with medium changes every three days. When cell confluence reached 80–90%, they were subcultured (passages 3–5) to ensure stability.
The characteristics of hUC-MSCs were confirmed by:
- Morphological examination: cells exhibited a spindle shape and were arranged in a swirling pattern resembling fibroblasts.
- Surface marker identification (flow cytometry): positive for CD73, CD90, and CD105; negative for CD34, CD45, and HLA-DR.
- Multipotent differentiation capacity: capable of differentiating into adipocytes, osteocytes, and chondrocytes in the respective induction media, confirmed by Oil Red O, Alizarin Red, and Alcian Blue staining.
Isolation and characterization of exosomes from hUC-MSCs
Exosomes (hUC-MSC-Exos) were collected from the conditioned medium of MSCs after 48 hours of culture in FBS-free medium. The collected medium was processed using a stepwise centrifugation protocol:
- First centrifugation: 300 × g for 10 minutes → to remove live cells.
- Second centrifugation: 2,000 × g for 20 minutes → to remove cell debris.
- Third centrifugation: 10,000 × g for 30 minutes → to remove large vesicles.
- Fourth centrifugation: 100,000 × g for 70 minutes (ultracentrifugation) → to pellet the exosomes.
The pellet was washed with PBS and re-centrifuged at 100,000 × g to further purify the exosomes, and then stored at −80 °C.
Exosome characteristics were confirmed using three techniques:
- Transmission electron microscopy (TEM): spherical vesicles with a double membrane, 80–150 nm in diameter.
- Nanoparticle Tracking Analysis (NTA): average diameter 100 ± 20 nm.
- Western blot: positive for characteristic proteins CD9, CD63, TSG101, and negative for calnexin (an intracellular marker, used as a control for contamination).
In vitro experiments on chondrocytes
Mouse articular chondrocytes were stimulated with IL-1β (10 ng/mL) to mimic the inflammatory conditions of OA. Subsequently, the cells were treated with hUC-MSC-Exos at different concentrations (10, 50, and 100 µg/mL).
Assessments included:
- Expression of inflammatory genes and proteins (IL-6, TNF-α, MMP13) measured by RT-qPCR and Western blot.
- Evaluation of cartilage-synthesizing genes (COL2A1, aggrecan) to determine the protective effect on cartilage tissue.
- Morphological analysis under fluorescence microscopy to assess the degree of chondrocyte degeneration.
Animal model (in vivo)
An OA model was established in mice by intra-articular injection of collagenase into the knee joint to induce cartilage degeneration. After 7 days, the mice were randomly divided into three groups:
- Group 1: Control (PBS)
- Group 2: Low-dose Exo (10 µg exosome per joint)
- Group 3: High-dose Exo (50 µg exosome per joint)
Intra-articular injections were administered once per week for 4 weeks. After the treatment period, the knee joints were harvested and assessed by
- Histological staining (HE and Safranin O/Fast Green) to evaluate cartilage structure.
• OARSI scoring (Osteoarthritis Research Society International) to quantify the degree of cartilage damage. - Histological analysis of the synovial membrane to evaluate inflammatory responses.
Early-phase clinical trial
Study design and participants
The phase I/IIa clinical trial was designed as a randomized, double-blind, dose-escalation study with a control group, aimed at evaluating the safety and preliminary efficacy of hUC-MSC-Exos in the treatment of knee osteoarthritis.
- Participants: 36 patients with OA of Kellgren–Lawrence grade II–III.
- Exclusion criteria: presence of other joint diseases, joint infection, coagulation disorders, or autoimmune diseases.
- Grouping:
- Low dose: 50 µg exosome per joint
- Medium dose: 100 µg per joint
- High dose: 200 µg per joint
- Control group: PBS solution
Exosomes were administered via intra-articular injection into the knee joint using a sterile fine needle, once per month for 3 months.
Follow-up and assessments
- Follow-up duration: 12 weeks after the final injection.
- Clinical evaluation:
- WOMAC (Western Ontario and McMaster Universities Osteoarthritis Index): to assess pain, stiffness, and physical function.
- VAS (Visual Analogue Scale): to assess subjective pain intensity.
- Imaging evaluation: MRI of the knee joint to analyze cartilage thickness, bone marrow edema, and synovial membrane status.
- Safety monitoring: complete blood count, liver and kidney function tests, CRP, local injection site reactions, and systemic adverse events.
All patients were closely monitored for the first 24 hours after injection to detect immediate reactions, and regular follow-up assessments were conducted throughout the 3-month study period.
Results
Preclinical findings:
- hUC-MSCs demonstrated successful differentiation into osteogenic, adipogenic, and chondrogenic lineages, confirming typical MSC characteristics.
- Isolated exosomes showed an average diameter of ~100 nm, spherical double-membrane morphology, and strong expression of surface markers CD9, CD63, and TSG101.
- In IL-1β–stimulated chondrocytes, hUC-MSC-Exos significantly downregulated IL-6 and MMP13 expression while upregulating COL2A1 and aggrecan, indicating anti-inflammatory and cartilage-protective effects.
- In the mouse OA model, intra-articular injection of exosomes reduced cartilage damage and synovial membrane thickening, as demonstrated by Safranin O staining and improved OARSI scores.
Clinical findings:
- No severe adverse reactions (e.g., anaphylaxis, infection, synovitis) were observed.
- Mild local swelling occurred in some patients at the injection site but resolved spontaneously within 1–2 days.
- After 12 weeks, WOMAC scores significantly decreased in the medium- and high-dose groups compared with the placebo group.
- MRI analysis revealed slight increases in cartilage thickness, reduced bone marrow edema signals, and improved joint tissue structure.
- No abnormalities were detected in liver enzyme levels, serum creatinine, or systemic inflammatory markers, confirming good overall safety.
Discussion
This study provides the first clinical evidence demonstrating that intra-articular injection of exosomes derived from hUC-MSCs is safe and can alleviate symptoms of osteoarthritis (OA) in humans.
Compared with direct stem cell–based therapies, exosome therapy offers several advantages:
- Non-cellular nature: lacks nuclei, eliminating the risk of tumor formation.
- Practical handling: easier to store, transport, and standardize in dosage.
- Minimally invasive: allows repeated administration without anesthesia or surgical procedures.
The proposed mechanisms of action include:
- Immunomodulation: exosomes suppress the NF-κB signaling pathway, reducing secretion of IL-1β and TNF-α.
- Chondroprotection: they enhance synthesis of collagen II and proteoglycans while inhibiting catabolic enzymes such as MMP13 and ADAMTS5.
- Tissue regeneration: exosomal microRNAs (e.g., miR-100-5p, miR-21, miR-140-3p) activate PI3K/AKT and Wnt/β-catenin signaling pathways, promoting chondrocyte proliferation.
MRI and WOMAC results showed the greatest improvement in the medium-dose group, suggesting an optimal therapeutic window. Higher doses may cause receptor saturation or increased phagocytic clearance of exosomes.
However, this study has certain limitations:
- The clinical sample size was relatively small, and follow-up duration was short (12 weeks).
- Long-term effects on cartilage structure and OA progression remain unclear.
- Further molecular analyses are required to identify key RNA and protein components responsible for therapeutic efficacy.
Conclusion
Exosomes derived from human umbilical cord mesenchymal stem cells (hUC-MSC-Exos) represent a promising, safe, and preliminarily effective therapy for knee osteoarthritis.
This study demonstrates that:
- Exosomes exhibit anti-inflammatory properties, protect chondrocytes, and promote joint tissue regeneration.
- Intra-articular injection of hUC-MSC-Exos is safe, with no serious adverse reactions observed.
- Clinical efficacy is reflected by significant improvements in WOMAC scores and MRI findings after 12 weeks.
The research team recommends conducting larger, multicenter, long-term clinical trials to confirm sustained efficacy and to optimize treatment protocols.
References
Source: Wang, Y., Kong, Y., Du, J., Qi, L., Liu, M., Xie, S., Hao, J., Li, M., Cao, S., Cui, H., Liu, A., Ma, J. & Song, Y. (2025). Injection of human umbilical cord mesenchymal stem cells exosomes for the treatment of knee osteoarthritis: from preclinical to clinical research. Journal of Translational Medicine, 23, 641.
Link: https://translational-medicine.biomedcentral.com/articles/10.1186/s12967-025-06623-y?utm_source=chatgpt.com#citeas