PLoS One, 06/05/2025
Introduction
Inflammation is an essential protective response of the body aimed at combating infectious agents and tissue damage [1]. However, the inflammatory response is also associated with various pathological conditions, including heart failure [2], osteoarthritis [3], rheumatoid arthritis [4], inflammatory bowel disease [5], diabetes [6], acute lung injury [7], acute kidney injury [8], and tissue fibrosis [9]. In addition, inflammation is thought to contribute to fatigue and is closely associated with the sensation of pain [10]. Given the central role of inflammation in these diseases, anti-inflammatory therapies have been investigated as potential treatment strategies for a range of different pathological conditions.
Mesenchymal stem cells (MSCs) are abundant adult stem cells with strong immunomodulatory and anti-inflammatory effects. MSCs act through:
- Secretion of anti-inflammatory cytokines (IL-10, TGF-β, IL-1RA).
- Modulation of immune cell activity → helping to balance the inflammatory response.
MSCs have been investigated in preclinical and clinical models for inflammation-related diseases. Sources of mesenchymal stem cells include:
- AD-MSCs (from adipose tissue):
- Effective in heart and lung diseases.
- Reduce myocarditis and promote tissue regeneration.
- Regulate macrophages and T cells → supporting ARDS treatment.
- UC-MSCs (from umbilical cord):
- Enhance renal tissue regeneration via paracrine effects.
- Exhibit stronger immunomodulatory and anti-inflammatory capabilities than AD-MSCs, suitable for renal regenerative therapy.
Many therapeutic effects of mesenchymal stem cells (MSCs) are thought to be mediated by secreted factors rather than the cells themselves, leading to conditioned medium from MSCs (MSC-CM) emerging as a potential alternative therapy [16]. Compared with direct MSC transplantation, MSC-CM therapy offers several advantages, notably a reduced risk of immune rejection, as MSC-CM does not contain live cells, thereby limiting inflammatory responses and eliminating the risk of immune-mediated graft rejection [17]. In addition, MSC-CM is considered safer from an oncological perspective, because while MSCs (endogenous or exogenous) can promote tumor cell proliferation under certain conditions, MSC-CM has shown tumor-suppressive effects by inhibiting oncogenic signaling, reinforcing its potential safety in patients at risk of cancer [18,19].
However, MSC-CM contains both cell-derived factors and components of the culture medium, which may influence safety and therapeutic efficacy. Historically, MSCs were cultured in media containing fetal bovine serum (FBS), making MSC-CM potentially contain animal-derived components and posing a risk of viral contamination. Furthermore, although some clinical reports have used MSC-CM in various diseases [20–22], efficacy and safety are considered dependent on the source material and production process, preventing generalization of results for human applications.
To address these concerns, the research team minimized the risk of contamination by obtaining adipose tissue or umbilical cords from healthy donors free of infectious diseases and culturing the cells in animal component-free media. MSC-conditioned medium (MSC-CM) was then selected after undergoing rigorous quality control, including viral safety testing of the final product. Additionally, the study compiled and evaluated the safety of relatively high-dose MSC-CM administration through various routes in four independent observational studies.
Materials and Methods
Ethical considerations
The treating physicians fully explained the study details to the participants through an informed consent document, and voluntary written consent was obtained prior to treatment.
Participants
The study included patients who did not achieve the desired outcomes with standard treatments, those who declined conventional medications due to concerns about side effects or other adverse factors, and cases for which physicians determined that treatment with MSC-CM was appropriate.
- Inclusion Criteria:
Patients aged 18 years or older who provided informed consent to participate, fully understood the treatment through explanatory materials, and voluntarily signed the consent form after thorough consultation. Only patients deemed necessary and suitable for treatment by the treating physician were included in the study.
- Exclusion Criteria:
Individuals with a history or suspected cognitive impairment, drug or stimulant use, pregnancy or breastfeeding, or those assessed by the treating physician as unsuitable for participation were excluded from the study.
- Additional Disease-Specific Inclusion Criteria:
- Patients with only pulmonary disease, including diffuse bronchiolitis, interstitial lung disease, or chronic obstructive pulmonary disease (COPD);
- Patients experiencing dyspnea due to lung disease and assessed by a physician as suitable for treatment;
- Patients with only cardiac disease, with serum NT-proBNP levels ≥ 900 pg/mL;
- Patients with isolated chronic kidney disease, with an estimated glomerular filtration rate (eGFR) ≤ 40 mL/min/1.73 m².
MSC-CM preparation
Conditioned medium from human mesenchymal stem cells derived from adipose tissue (AD-MSC-CM) and umbilical cord (UC-MSC-CM) was produced in animal component-free media, following a manufacturing process carried out by BioMimetics Sympathies, Inc. (Tokyo, Japan). Tissue samples for AD-MSC-CM and UC-MSC-CM were obtained from two Japanese women in their 20s, who tested negative for viruses and provided written consent for donation.
From these samples, mesenchymal stem cells (AD-MSCs and UC-MSCs) were isolated, and the stromal fraction was cultured in serum-free MS-E0001 medium (BioMimetics Sympathies, Inc., Tokyo, Japan) at 37 °C under 5% CO₂. Subsequently, 1.2–1.8 × 10⁶ MSCs at passages 4–5 were cultured in 28–35 mL of MS-E0001 medium. When cell confluence reached 90%, the medium was replaced with MS-E0006 medium (BioMimetics Sympathies, Inc.) and incubated for an additional 2 days.
The conditioned medium (MSC-CM) was then collected and filtered through a 0.22 µm membrane. During production, viral contamination testing was performed, and the final product passed endotoxin, sterility, mycoplasma, and color assessments. Visual inspection for foreign material contamination was also conducted, with no abnormalities detected. Finally, MSC-CM was aliquoted and stored at –30 °C until use. Both AD-MSC-CM and UC-MSC-CM used in this study were derived from the same respective donor (each type of MSC-CM came from a single donor).
Enzyme-linked immunosorbent assay
The concentrations of HGF and exosomes in the conditioned medium (CM) were measured using ELISA according to the manufacturer’s instructions.
- HGF: assessed for its ability to promote cell proliferation, migration, and differentiation → reflecting regenerative therapeutic efficacy.
- Exosomes: evaluated for their role in signaling and cellular recovery.
Human umbilical vein endothelial cell assay
Experiments on human umbilical vein endothelial cells (HUVECs) were conducted to evaluate the anti-inflammatory effects of AD-MSC-CM and UC-MSC-CM. The aim was to determine whether MSC-CM could reduce TNF-α-induced inflammatory responses in endothelial cells.
HUVECs were cultured in DMEM/F12 medium supplemented with 10% fetal bovine serum (FBS), 10 ng/mL basic fibroblast growth factor (b-FGF), and 10 μg/mL gentamicin at 37 °C under 5% CO₂. Once cells reached an appropriate density, inflammation was induced by adding 10 ng/mL TNF-α to the culture medium. Simultaneously, experimental groups were treated with control medium (MS-E0006), adipose-derived MSC-conditioned medium (AD-MSC-CM), or umbilical cord-derived MSC-conditioned medium (UC-MSC-CM) at a final concentration of 75%.
Cells were incubated for 3 days, then lysed to extract RNA. The RNA was reverse-transcribed into cDNA. Real-time PCR was performed to determine the expression levels of IL-6 and IL-8, two hallmark inflammatory markers. GAPDH was used as the housekeeping gene to normalize gene expression data.
Administration schedule
Prior to initiating treatment, patients were interviewed approximately 1 week before the first infusion. For intra-arterial (IA) and intravenous (IV) administration, the conditioned medium (CM) was administered three times per month. For inhalation (INH), CM was administered once per week, for a total of 12 treatments.
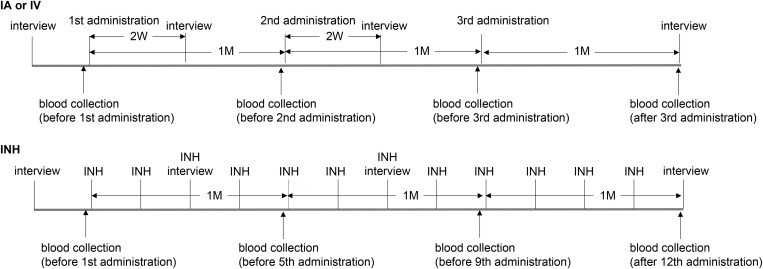
Upper panel: Participants’ schedule for intra-arterial (IA) or intravenous (IV) administration. Patients were interviewed approximately one week prior to the first administration, and the CM was administered three times per month. Blood samples were collected immediately before each administration and one month after the third administration, with serum stored. Interviews about changes in subjective symptoms were conducted two weeks after the first and second administrations, and one month after the third administration.
Lower panel: Participants’ schedule for inhalation (INH) administration. Patients were interviewed approximately one week before the first administration, and 12 administrations were conducted weekly. Blood samples were collected immediately before the first, fifth, and ninth administrations, and one week after the 12th administration, with serum stored. Interviews were conducted before the third and seventh administrations, and one week after the 12th administration.
Administration methods
- Basic policy
For diseases with clearly affected organs (heart, lungs, kidneys), CM was administered in a large volume (≈30 mL) over 24 hours via the arterial route to achieve effective local concentration at the diseased site.
For the treatment of fatigue (chronic), CM was administered intravenously (≈10 mL) for systemic distribution, reducing costs and ensuring safety by infusing slowly over 3 hours.
- Lung disease and heart failure
For patients with pulmonary disease or heart failure, 30 mL of adipose-derived MSC-conditioned medium (AD-MSC-CM) was mixed with 500 mL of physiological saline. The mixture was infused into the pulmonary artery via the median cubital vein using a Swan-Ganz catheter over 24 hours.
If no improvement in subjective clinical symptoms was observed after the first infusion, the patient received UC-MSC-CM (umbilical cord-derived MSC-conditioned medium) in the subsequent session. In cases where catheter placement was difficult, CM was administered intravenously over 3 hours.
- Chronic kidney disease
For patients with chronic kidney disease, 30 mL of umbilical cord-derived MSC-conditioned medium (UC-MSC-CM) was mixed with 500 mL of physiological saline. The mixture was administered via a Good Tech HT catheter placed in the median cubital vein, directed into the descending aorta approximately 2 cm above the branching point of the renal arteries, and infused continuously over approximately 24 hours.
- General fatigue
For patients experiencing whole-body fatigue, 10 mL of adipose-derived MSC-conditioned medium (AD-MSC-CM) was mixed with 500 mL of physiological saline and administered via the median cubital vein over approximately 3 hours. If no significant improvement in subjective symptoms was observed, the patient received UC-MSC-CM in the subsequent infusion.
In cases where intravenous administration was not feasible, patients received inhalation (INH) therapy using a mixture of 1 mL AD-MSC-CM and 4 mL physiological saline via a nebulizer. Inhalation therapy was performed once per week for a total of 12 sessions.
Interviews
Prior to infusion, the treating physician discussed current symptoms and medical history with the patient.
For intra-arterial (IA) and intravenous (IV) administration, interviews were conducted 2 weeks after the first and second infusions, as well as 1 month after the third infusion, to assess subjective symptoms and any adverse effects.
For inhalation (INH) therapy, the physician interviewed patients on the days of the third and seventh inhalation sessions, and 1 week after the twelfth (final) session, to monitor condition and treatment responses.
Blood tests
Serum samples were collected before each infusion and 1 month after the third infusion (for inhalation [INH]: before the first, fifth, and ninth sessions, and 1 week after the twelfth session). Necessary tests were conducted according to each patient’s specific disease. Although mesenchymal stem cells (MSCs) have immunomodulatory effects, administration of MSC-conditioned medium (MSC-CM) may induce unintended inflammatory or allergic responses. Therefore, monitoring patients’ inflammatory markers throughout treatment is crucial.
To assess inflammatory status, the research team quantified serum C-reactive protein (CRP) levels before and after MSC-CM administration. CRP is a protein rapidly produced by the liver in response to inflammation in the body. While test items were specified for each study depending on the disease, CRP was a common marker measured in all patients.
Statistical analyses
CRP data were assessed using the Kolmogorov–Smirnov test and analyzed with the Wilcoxon test due to non-normal distribution. Analyses were performed in R 4.4.1, with a significance threshold of p < 0.05.
Results
Analyses of the features of AD- and UC-MSC-CM
To compare the properties of adipose-derived MSC-conditioned medium (AD-MSC-CM) and umbilical cord-derived MSC-conditioned medium (UC-MSC-CM), the research team quantified hepatocyte growth factor (HGF) and exosome numbers using ELISA. The results showed no significant differences in HGF concentration or exosome quantity between the two types of conditioned media.
Next, the anti-inflammatory effects of MSC-CM were evaluated on human umbilical vein endothelial cells (HUVECs). The results demonstrated that both AD-MSC-CM and UC-MSC-CM suppressed TNF-α-induced mRNA expression of IL-6 and IL-8, indicating that both types of MSC-CM exhibit pronounced anti-inflammatory activity.
Safety tests
A total of 55 participants were enrolled in the study (31 men, 24 women; mean age 77.4 ± 10.1 years). The distribution by disease was as follows: pulmonary disease (12 participants; 7 men, 5 women), heart failure (14 participants; 7 men, 7 women), chronic kidney disease (10 participants; 7 men, 3 women), and whole-body fatigue (19 participants; 10 men, 9 women). Among them, 54 participants received three intravenous infusions, and 1 participant received 12 inhalation (INH) treatments, totaling 174 MSC-CM administrations.
Several mild adverse events were observed, including:
- Dizziness during massage (SK003, after the first infusion; CTCAE v5.0 code 1001375, grade 1)
- Loss of appetite (SK004, after the second infusion; CTCAE code 10002646, grade 1)
- Enterocolitis (SH008, after the third infusion; CTCAE code 10014893, grade 1)
- Post-renal hydronephrosis (SH008, after the third infusion; CTCAE code 10038369, grade 2)
- Fever of 40 °C (SH009, after the first infusion; CTCAE code 10016558, grade 2).
No serious adverse events, such as death, life-threatening conditions, significant disability, permanent injury, or the need for emergency medical intervention, were reported during the 174 administrations. Among the 55 patients, 4 experienced mild reactions with unclear causality related to MSC-CM administration.
Changes in inflammatory markers
There was no statistically significant difference in serum CRP levels before and after administration of either AD-MSC-CM or UC-MSC-CM (p = 0.5994, n = 55). Although CRP slightly decreased post-infusion, these results suggest that MSC-CM administration does not induce significant inflammatory responses in participants.
The researchers compared changes in CRP levels before and after MSC-CM administration according to the route of administration. CRP tended to decrease across all routes (IA, IV, INH), although the differences were not statistically significant. Notably, the single patient treated via inhalation (INH) showed a more pronounced reduction in CRP. An exception was patient SH008, who developed unexplained colitis after the third infusion and was subsequently diagnosed with post-renal hydronephrosis. The relationship between this event and MSC-CM administration remains undetermined.
Due to high variability in baseline CRP values, samples were divided into two groups:
- CRP > 0.3 mg/dL (inflamed): CRP decreased significantly after infusion (1.109 → 0.413 mg/dL, p = 0.0122) → suggesting that MSC-CM exerts anti-inflammatory effects in participants with elevated inflammation.
- CRP < 0.3 mg/dL (normal): CRP increased slightly but remained within the normal range (0.069 → 0.098 mg/dL, p = 0.0415) → no new inflammatory response was induced.
Discussion
The primary objective of this study was to evaluate the safety of multiple administrations of large-volume mesenchymal stem cell-conditioned medium (MSC-CM) (10 or 30 mL). The results showed no significant differences in composition or effects between adipose-derived MSC-CM (AD-MSC-CM) and umbilical cord-derived MSC-CM (UC-MSC-CM), making it premature to determine which type is more suitable for specific diseases.
Across a total of 162 intravenous infusions in 54 patients and 12 inhalation sessions in 1 patient, no serious adverse events were observed. This suggests that the components of MSC-CM may not trigger excessive immune activation, supporting its safety for repeated use.
Four patients in the study experienced mild adverse effects, including loss of appetite, dizziness, and transient fever. Two patients with chronic kidney disease who received UC-MSC-CM via the descending aorta reported loss of appetite and dizziness, while a patient with heart failure who received AD-MSC-CM via the pulmonary artery experienced a temporary fever. These symptoms occurred only once during the three infusions and resolved spontaneously within 1–2 days without medical intervention. Furthermore, CRP levels in these patients either decreased or remained within the normal range (<0.3 mg/dL), suggesting that MSC-CM was unlikely to be the direct cause of these reactions.
Another patient (SH008) with an initial CRP above 0.3 mg/dL showed an increase in CRP after receiving AD-MSC-CM via the pulmonary artery. Two days after the third infusion, the patient was diagnosed with unexplained colitis followed by post-renal hydronephrosis, although these events were unlikely to be directly related to the administration route or MSC-CM. While the overall results indicate that MSC-CM therapy is safe and well-tolerated with repeated use, further studies with larger sample sizes are needed to confirm long-term safety and elucidate potential mechanisms of action.
This study evaluated the safety of systemic administration of mesenchymal stem cell-conditioned medium (MSC-CM) by monitoring serum CRP fluctuations. The results showed an overall decrease in mean CRP among all 55 patients, with a statistically significant reduction in the group with CRP ≥ 0.3 mg/dL, suggesting that MSC-CM may help suppress systemic inflammatory responses. In the group with CRP < 0.3 mg/dL, CRP increased slightly but remained within the normal range, without clinical significance.
All observed adverse effects were mild, transient, and self-resolving, with no serious events reported across 174 administrations, indicating that MSC-CM therapy is safe, including in elderly patients (mean age 76.2 ± 11.1 years). These findings confirm the tolerability and therapeutic potential of MSC-CM for chronic diseases and highlight its promise as a safer biological treatment option.
Notably, this study is among the few to report the repeated administration of large-volume MSC-CM (10–30 mL) via arterial, intravenous, or inhalation routes while maintaining safety. No significant differences in CRP reduction were observed between administration routes, suggesting that intravenous or inhalation delivery may be more practical for broader use. However, given the limited number of cases—especially for the inhalation route—future studies should include larger sample sizes and evaluate additional markers such as Galectin-3, NT-proBNP, or eGFR to more comprehensively assess therapeutic efficacy.
Conclusion
This study confirms the safety of repeated administration of mesenchymal stem cell-conditioned medium (MSC-CM) produced using animal component-free technology in 55 patients with chronic diseases unresponsive to conventional treatments. No serious adverse events were observed, and mild side effects occurred only in a few cases.
Changes in serum CRP levels indicated that systemic MSC-CM administration did not trigger inflammatory responses, demonstrating that this therapy is well-tolerated and safe. These findings suggest that properly manufactured and administered MSC-CM may serve as a potential treatment option for chronic diseases, offering a novel approach for clinicians and patients. However, further large-scale studies are needed to fully evaluate the therapeutic efficacy and underlying mechanisms of MSC-CM.
References
Source: Inami N. (2025). Safety assessment of multiple systemic administration of human mesenchymal stem cell-conditioned medium for various chronic diseases. PLoS One. May 6;20(5): e0322497.








