Journal of Clinical Oncology, April 15, 2025
Abstract
A multicenter, randomized clinical trial conducted in China demonstrated that sequential infusion of umbilical cord–derived mesenchymal stem cells (UC-MSCs) within 3 months following haploidentical hematopoietic stem cell transplantation (haplo-HSCT) significantly reduced the incidence and severity of graft-versus-host disease (GVHD), while markedly improving GVHD-free and relapse-free survival (GRFS).
Background
Haplo-HSCT has become increasingly prevalent in the treatment of hematologic malignancies in China. However, GVHD remains one of the leading complications affecting patient survival and quality of life. Mesenchymal stem cells (MSCs) possess strong immunomodulatory properties, but the prophylactic efficacy of MSCs against GVHD largely depends on the timing and frequency of administration.
Study Design and Methods
- Study type: Multicenter, randomized, open-label clinical trial
- Participants: 192 patients (aged 18–60 years) undergoing haplo-HSCT
- Study arms:
- MSC group: Received 8 doses of UC-MSCs (1×10⁶/kg), starting 4 hours before transplantation (day 0) and continuing over a 3-month schedule
- Control group: Received standard GVHD prophylaxis (mycophenolate mofetil, cyclosporine A, methotrexate)
- Primary endpoint: 2-year cumulative incidence of severe chronic GVHD (cGVHD)
- Secondary endpoints: Incidence of acute GVHD (aGVHD), GVHD-free and relapse-free survival (GRFS), relapse rate, non-relapse mortality, and adverse events
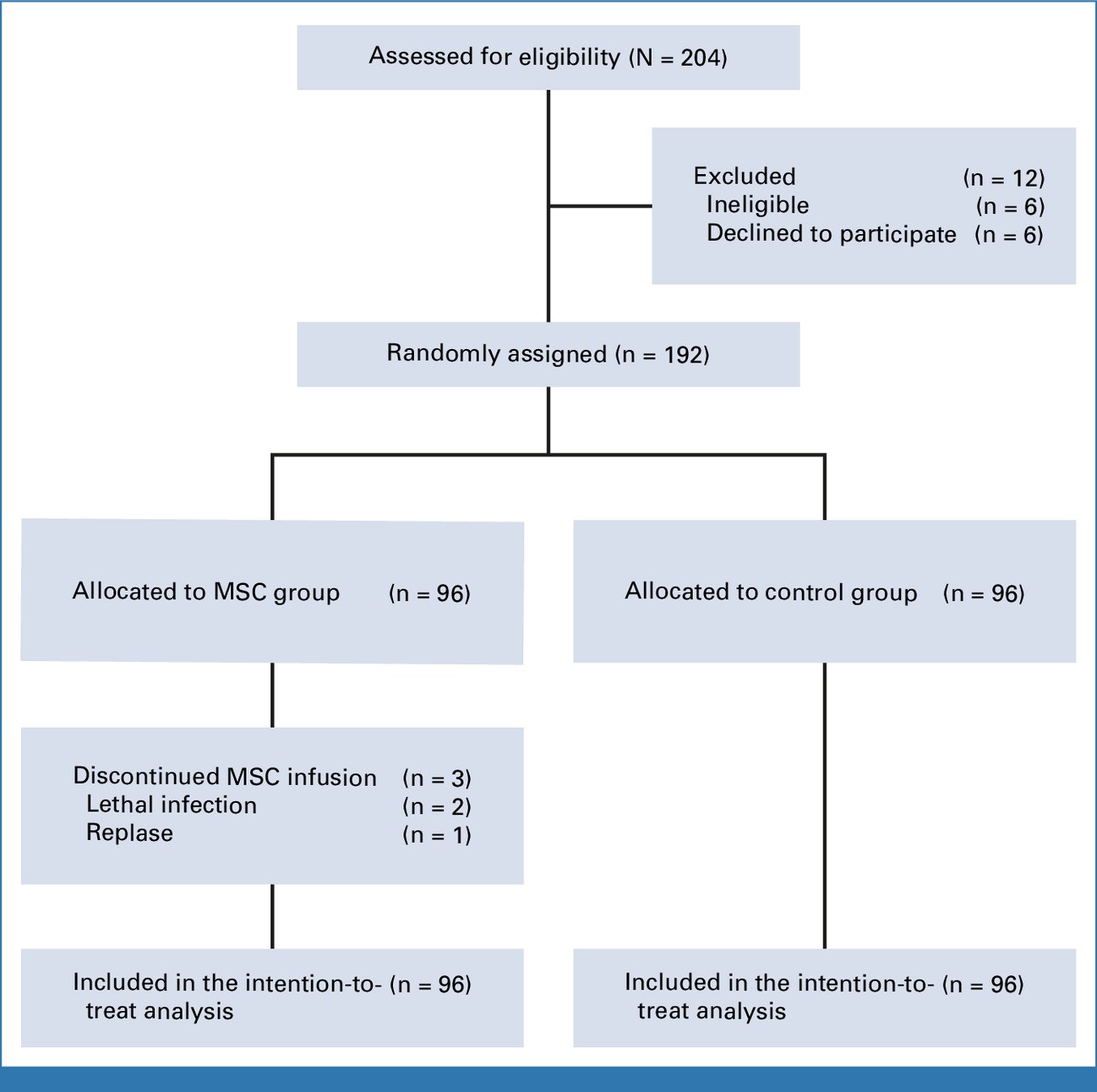
Figure 1: Experimental Design Schematic
Key Findings
- Significant reduction in GVHD incidence:
- Severe cGVHD: 5.5% (MSC group) vs. 14.8% (control group), P = 0.033
- Grade 2–4 aGVHD: 4.2% vs. 30.2%, P < 0.001
- Grade 3–4 aGVHD: 2.1% vs. 21.9%, P < 0.001
- Improved GRFS:
- 62.4% in the MSC group vs. 32.0% in the control group, P < 0.001
- No increased risk of relapse or non-relapse mortality:
- Relapse: No significant difference, P = 0.34
- Non-relapse mortality: No significant difference, P = 0.45
- Target organ protection:
MSC infusion significantly reduced cGVHD-related damage to skin, lungs, and eyes.
Safety
- No adverse events directly related to MSC infusion were reported
- Two deaths occurred due to infection, but were unrelated to MSC treatment
- EBV and CMV reactivation rates were similar in both groups (~45%)
- Lower incidence of grade 3–4 hemorrhagic cystitis in the MSC group (P = 0.031)
Conclusion
Sequential UC-MSC infusion following haplo-HSCT represents an effective and safe strategy for GVHD prophylaxis, offering significant improvements in prognosis and quality of life. This approach should be considered for incorporation into standard care protocols for haplo-HSCT recipients in the future.
References
The article was translated and summarized from the research paper: Yao, H., Huang, R., Fu, H., Lin, R., Zhang, Y., Feng, Y., Wang, Y., Chen, T., Wang, X., Zhu, L., Liu, J., Liu, Y., Zhao, L., Wang, L., Kong, P., Wen, Q., Zhang, C., Gao, L., Gao, L., Liu, Q., … Zhang, X. (2025). Sequential Infusion of Mesenchymal Stem Cell for Graft-Versus-Host Disease Prevention in Haploidentical Hematopoietic Stem Cell Transplantation: An Open-Label, Multicenter, Randomized Controlled Clinical Trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology, JCO2402119. Advance online publication. https://doi.org/10.1200/JCO-24-02119
Source: Journal of Clinical Oncology
Link: https://doi.org/10.1200/JCO-24-02119








