Frontiers in Immunology, 17/06/2025
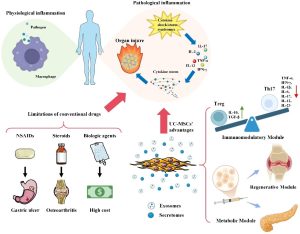
A new original study published in Frontiers in Immunology (Volume 16, 2025) reports preliminary findings on the safety and efficacy of human umbilical cord-derived mesenchymal stem cells (hUC-MSCs) in the treatment of critically ill patients with COVID-19.
Study Objective
To evaluate the safety and potential of hUC-MSCs to improve immune function and COVID-19-related pneumonia through the administration of three intravenous doses in critically ill patients.
Methods
- Participants: Five patients with severe COVID-19 who were unresponsive to standard treatments.
- Dosage: Intravenous infusion of three doses of hUC-MSCs at 3 × 10⁷ cells per dose, administered every two days.
- Primary Assessments:
- Clinical progression: Inflammatory storm, improvement of lung lesions on CT scans, and oxygenation levels.
- Biomarkers: D-dimer, CRP, IL-6, leukocyte subpopulations (CD4, CD19, CD16+56, etc.).
- Overall immune function and safety, with no serious adverse events observed.
Key Results
| Outcome | Observed Effect |
| Safety | No serious adverse events reported after infusion |
| Radiological findings | Significant improvement in lung CT scans |
| Immunological response | Increased lymphocyte counts, decreased CRP and IL-6 |
| Oxygenation function | Improved oxygenation post-infusion |
Conclusion
- hUC-MSCs are safe for intravenous infusion in critically ill COVID-19 patients.
- The study shows promising signs of improved inflammatory control, immune modulation, and pulmonary recovery, highlighting the regenerative potential of this therapy — especially given the lack of effective treatments for diffuse alveolar damage caused by SARS-CoV-2.
Significance and Future Perspectives
- This is one of the first clinical trials to investigate the use of umbilical cord-derived stem cells in the treatment of severe COVID-19, aiming to reduce inflammation and restore immune function.
- The encouraging safety profile provides a strong basis for larger-scale clinical trials to explore the therapy’s true efficacy — particularly in the context of limited options for treating severe pulmonary injury.
- In the future, hUC-MSCs may also be evaluated in other causes of severe inflammatory or viral respiratory failure, beyond COVID-19.
Reference
This summary is based on the provisionally accepted article:
Yao, W., Li, B., Jiang, Y., Yuan, Q., Wu, W., Hou, R., Qi, Q., Dong, H., Zhang, Y., & Zhang, Y. (2025). The salvage therapy utilizing human umbilical cord-derived mesenchymal stem cells for the treatment of critically ill patients with COVID-19. Frontiers in Immunology, 16. https://doi.org/10.3389/fimmu.2025.1594373
Source: Frontiers in Immunolog
Link: https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2025.1594373/abstract