Biomolecule, 10 April 2025
Background
Stroke is a leading cause of death and disability worldwide, with ischemic stroke accounting for up to 87% of all cases. Current treatment options (such as the thrombolytic agent tPA) offer limited efficacy, are highly time-dependent, and cannot fully restore damaged neural tissue.
The Role of Mesenchymal Stem Cells (MSCs)
Mesenchymal Stem Cells (MSCs) are a type of adult stem cell with the ability to:
- Differentiate into various neural cell types
- Modulate immune responses and reduce inflammation
- Stimulate new blood vessel formation
- Reduce cell death and promote neural regeneration
MSCs can be derived from various sources:
- Bone Marrow (BM-MSCs)
- The classical and most extensively studied source.
- Advantages: Rich in highly active MSCs with good differentiation capacity.
- Disadvantages: Invasive collection, painful and requires anesthesia. MSC quantity and quality decline with age, making it suboptimal for elderly or comorbid patients.
- Has been used in many clinical trials for stroke treatment (e.g., SB623 product).
- Adipose Tissue (AT-MSCs)
- Harvested via subcutaneous liposuction; less invasive than bone marrow aspiration.
- Contains a higher density of MSCs compared to bone marrow.
- Strong differentiation capacity, especially toward neural and vascular tissues.
- Easy to collect, suitable for personalized therapy development.
- Dental Pulp (DP-MSCs)
- Obtained from exfoliated deciduous teeth or extracted wisdom teeth — non-invasive.
- MSCs from dental pulp originate from the neural crest, thus have high neurogenic potential.
- Promising for neurological disorders, though clinical applications remain limited.
- Umbilical Cord (UC-MSCs)
- An abundant and non-invasive source, usually discarded after birth.
- UC-MSCs are younger and less affected by donor age, with high proliferation and low immunogenicity — ideal for off-the-shelf therapies.
- Exhibit strong anti-inflammatory properties, favored in treating neurodegenerative, inflammatory, and autoimmune conditions.
- Umbilical Cord Blood (UCB-MSCs)
- Rarer than UC-MSCs but still a valuable MSC source.
- Possess good differentiation and immunomodulatory potential, though present in lower quantities — requiring expansion in culture.
- Mainly used in research or in combination with cord blood therapy for stroke or cerebral palsy.
- Placenta & Amniotic Membrane
- These perinatal tissues are rich in MSCs with low immunogenicity and high tissue regeneration capability.
- Increasingly utilized due to their availability, non-invasive harvesting, and low cost.
- Some studies have explored AM-MSCs (amniotic MSCs) for hypoxic brain injury and neuroinflammation.
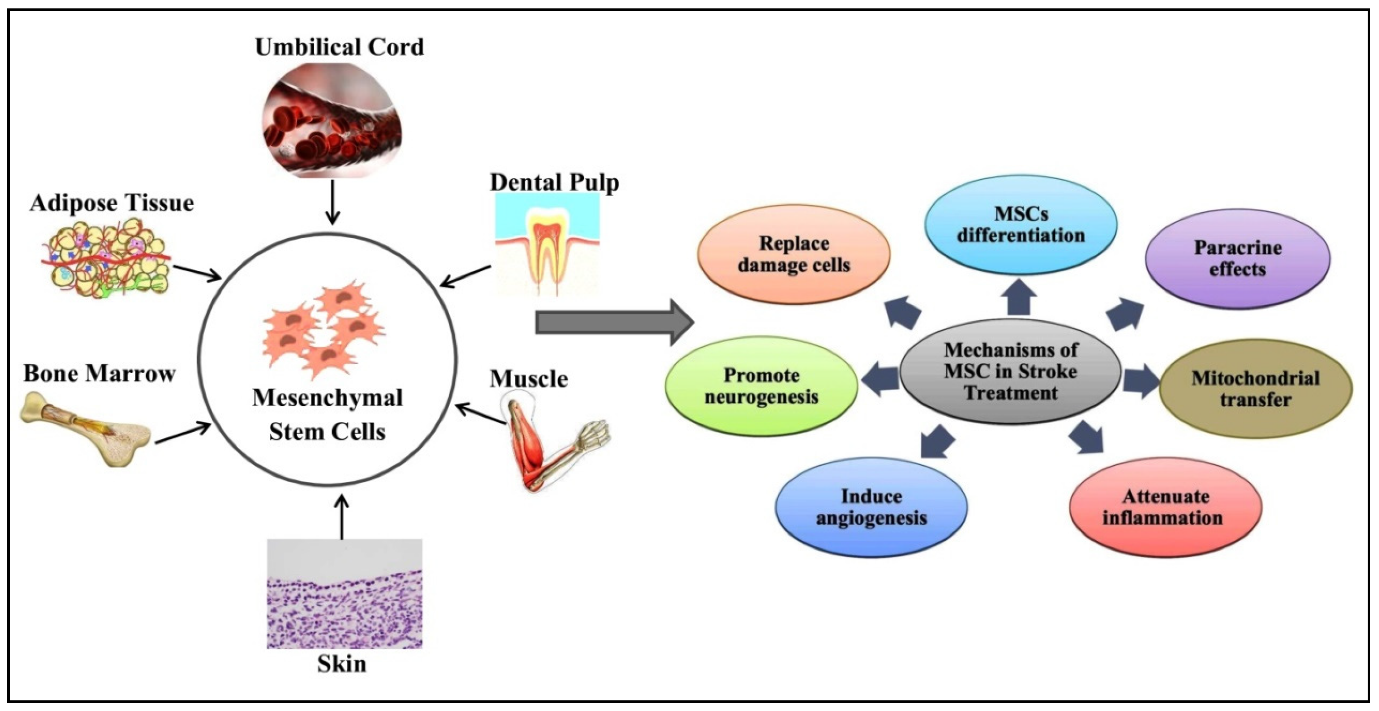
Figure 1. Sources and therapeutic mechanisms of mesenchymal stem cells (MSCs). MSCs can be isolated from the umbilical cord, adipose tissue, bone marrow, skin, muscle, and dental pulp. MSCs can then be employed in stroke treatment. The therapeutic role of MSCs is due to different mechanisms, including direct differentiation into neuronal cells and tissues, paracrine effects, mitochondrial transfer, inflammation attenuation, angiogenesis, neurogenesis, and the removal of damaged brain cells.
Mechanisms of MSCs in Stroke Therapy
- Direct differentiation into neurons and glial cells
- Paracrine effects: secretion of cytokines, exosomes, and growth factors
- Mitochondrial transfer to restore function of damaged cells
- Immunomodulation: reduction of pro-inflammatory cytokines such as TNF-α, IFN-γ, MCP-1, etc.
- Promotion of angiogenesis and neurogenesis
- Brain tissue regeneration and enhancement of neural connectivity
Notable Clinical Trials
- As of August 2024, 27 clinical trials involving MSCs in stroke treatment have been registered.
- Most trials utilized MSCs from umbilical cord or bone marrow, administered intravenously or intracranially.
- A notable study: SB623 (genetically modified BM-MSCs) showed safety and improved motor function in patients with chronic stroke (NCT01287936).
Challenges and Future Directions
- Timing of cell delivery: Highest efficacy when administered within 48 hours, but difficult to obtain sufficient MSCs quickly.
- Heterogeneity in cell sources, dosage, and delivery methods affects outcome consistency.
- Limited ability of MSCs to cross the blood-brain barrier (BBB).
- Influence of donor age, comorbidities, and concurrent medications on MSC efficacy remains underexplored.
Conclusion
MSC therapy holds significant promise in stroke treatment – not only supporting brain tissue recovery but also reducing inflammation and enhancing motor function. However, for widespread clinical application, further standardized phase III trials and strategies to enhance MSC bioefficacy, especially in elderly and comorbid patients, are essential.
References
The article was translated and summarized from the research paper: Choudhery MS, Arif T, Mahmood R, Harris DT. Therapeutic Potential of Mesenchymal Stem Cells in Stroke Treatment. Biomolecules. 2025; 15(4):558. https://doi.org/10.3390/biom15040558
Source: Biomolecules








