Cureus, 08/08/2025
Background
Neurodegenerative diseases (NDD), including Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis (ALS), and spinal cord injury (SCI), cause neuronal loss, chronic inflammation, demyelination, and disruption of synaptic networks. Current treatments mainly slow disease progression or control symptoms; the need for neural tissue regeneration remains unmet.
Stem cell (SC) therapy has emerged with three main strategies:
- Cell replacement
- Neuroprotection / immunomodulation
- Remyelination and stimulation of axonal regeneration
Overview
- Diverse cell sources
- MSC (Mesenchymal stem cell) from bone marrow, adipose tissue, umbilical cord
- NSC (Neural stem cell)
- OPC (Oligodendrocyte progenitor cell)
- iPSC (Induced pluripotent stem cell)
- ESC (Embryonic stem cell)
- Multimodal mechanisms
- Secretion of trophic factors
- Regulation of the inflammatory microenvironment
- Activation of endogenous repair
- Functional integration as new neurons or oligodendrocytes
- Routes of administration & biomaterial scaffolds
- Direct intraparenchymal/injury site injection
- Intrathecal injection
- Intravenous infusion
- Transplantation with biomaterial scaffolds/hydrogel to enhance cell survival and localization
- Early clinical evidence
- Phase I/II trials in SCI, PD (Parkinson’s disease), MS (Multiple sclerosis), ALS show safety, feasibility, and some efficacy signals (motor/sensory improvement, functional score gains)
- No robust Phase III evidence yet to alter standard treatment protocols
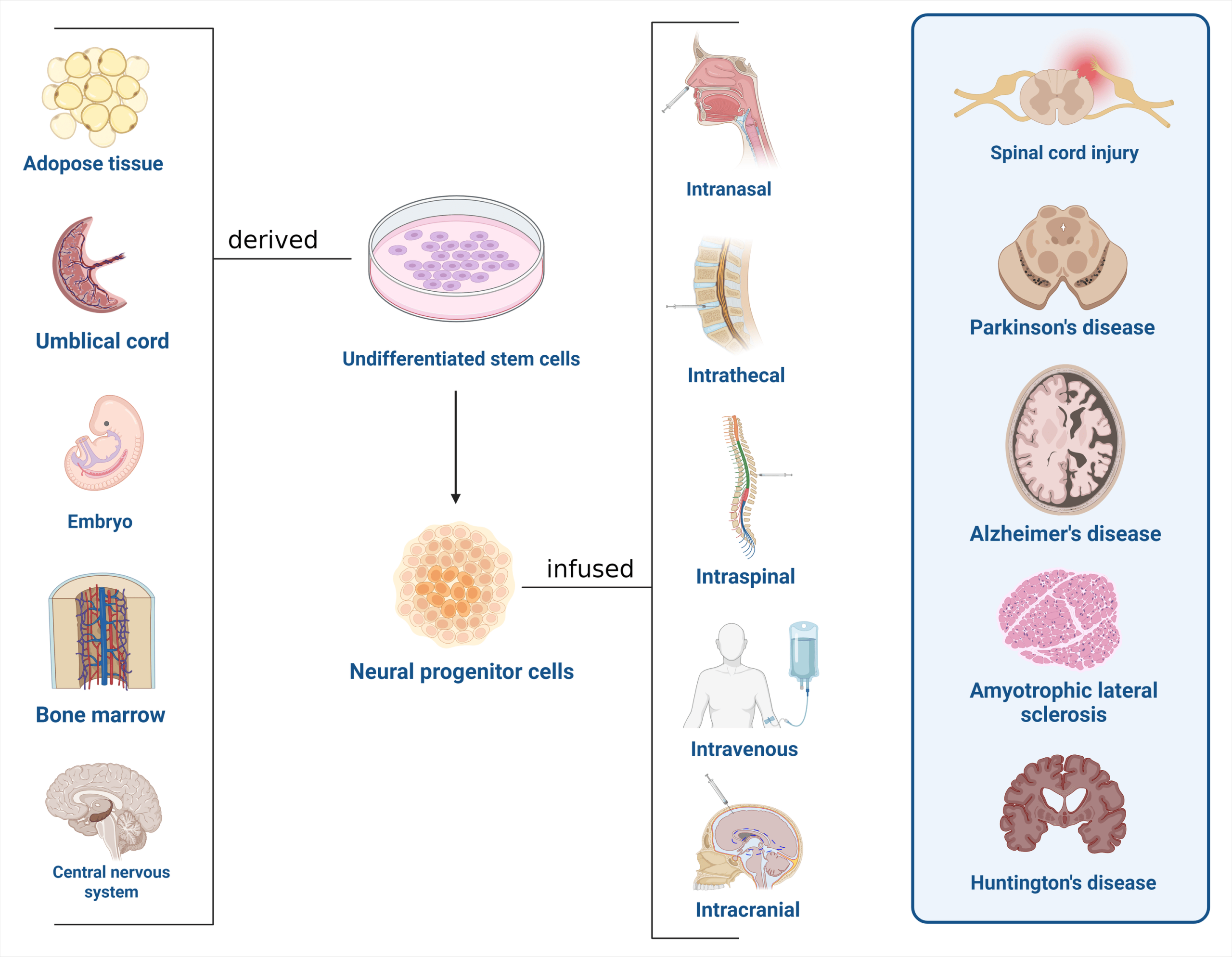
Figure 1: Overview of stem cell therapy in neurodegenerative diseases and spinal cord injury
Table 1: Summary of human trials of stem cell therapy for Alzheimer’s disease.
| Author (Year) | No. of participants | Type of stem cells | Mode of delivery | Patient outcomes |
| Kim et al (2021) | 9 | human umbilical cord blood-derived mesenchymal stem cells (hUCB-MSCs) | Injections to right lateral ventricles via Ommaya reservoir | Therapy was feasible, relatively and sufficiently safe, and well tolerated. |
| Kim et al. (2015) | 9 | Umbilical cord derived stem cell. | Stereotactic injection | No adverse reactions reported other than headache and wound pain. |
| Rash et al. (2025) | 120 | Bone marrow derived stem cells. | Intravenous infusions. | Improvement in cognitive tests among patients. Reduction in neuroinflammation. |
| Brody et al. (2023) | 33 | Allogeneic mesenchymal stem cells. | Intravenous infusion. | Subjects in low dose group demonstrated improvement in cognitive tests. |
Table 2: Summary of human trials of stem cell therapy for Parkinson’s disease and amyotrophic lateral sclerosis.
| Author (Year) | Disease | No. of participants | Type of stem cells | Mode of delivery | Patient outcomes |
| Jiang S et al. (2018) | PD | 18 | ANGE-S003 human neural stem cells | Intranasal transplantation | Improved performance on MDS-UPDRS with peak at 6th month with no serious adverse effects |
| Barker et al. (2019) | PD | 11 | Neural Allo-Transplantation with Fetal Ventral Mesencephalic Tissue | Surgical injection (transplantation). | Grafted cells can survive over 10 years, reduced bradykinesia and rigidity. Sustained re-activation of motor cortical areas. |
| Berry et al. (2019) | ALS | 36 | mesenchymal stem cell (MSC)-neurotrophic factor (NTF) cells | combined intrathecal and intramuscular administration | Safe and early promising signs of efficiency. |
| Barczewska Monika et al. (2020) | ALS | 67 | Wharton’s jelly mesenchymal stem cells | Intrathecal administration | The stem cells proved safe and efficacious. Female participants responded better. |
| Siwek et al. (2020) | ALS | 8 | Autologous bone marrow-derived mesenchymal stem cells | Intrathecal administration | Disease progression decreased in patients with rapid course ALS. |
| Mazzini et al. (2019) | ALS | 18 | Human neural stem cell | Micro transplantation into spinal cord | Decrease in disease progression with no side effects up to 60 months. |
| Alkhazaali-Ali et al. (2025) | ALS | 21 | Autologous bone marrow-derived mesenchymal stem cells | Intrathecally and intravenously concurrently | Steadiness in ALSFRS and FVC values. |
Table 3: Summary of human trials of stem cell therapy in spinal cord injury
| Author (Year) | Type of study | No. of participants | Type of stem cell administered | Mode of delivery | Patient outcomes |
| Akhlaghpasand et al. (2025) | Phase II randomized active- controlled trial. | 44 | MSCs and Schwann cells. | Intrathecal injection. | Significant improvement in neurogenic bladder function. Some patients also experienced secondary motor and sensory improvements. No serious adverse events were reported. |
| Awidi et al. (2024) | Phase I/II clinical trial. | 14 | Expanded MSCs from bone marrow and umbilical cord origins | Intrathecal injection. | Umbilical cord-derived MSCs showed slightly better outcomes compared to bone marrow-derived MSCs. No serious adverse events were observed. Some patients experienced improvements in motor function and bladder control. |
| Bydon et al. (2024). | Phase I clinical trial. | 10 | Adipose-derived mesenchymal stem cells (AD-MSCs). | Intrathecal injection | No serious adverse events were reported. Some participants showed improvement in motor and sensory scores, especially those with incomplete injuries. Neuropathic pain and quality of life measures also improved in a subset of patients. |
| Shang et al. (2022) | Systemic review | 2,399 patients across 62 clinical trials. | Mesenchymal stem cells (MSCs), and neural stem/progenitor cells (NSPCs). | Intrathecal injection, intraspinal injection, intravenous injection, and surgical implantation | 43.2% of patients showed at least one-grade improvement on the ASIA scale. Sensory function showed modest improvement. Mild to moderate adverse events were common; serious adverse events were rare. |
| Hur et al. (2016) | 14 | Autologous adipose-derived mesenchymal stem cells (ADMSCs). | Intrathecal administration via lumbar tapping. | Motor and sensory function improved in patients with incomplete spinal cord injury. No severe adverse events were reported. Improvements were observed in neuropathic pain and bladder function in some cases |
Limitations and Challenges
- Heterogeneity in Cell Types and Protocols
- There is currently no standardized guideline for selecting specific stem cell types for each neurological condition.
- Studies vary in cell sources, differentiation protocols, and delivery routes, making results difficult to compare.
- Long-Term Safety Risks
- Risk of tumor formation, particularly with induced pluripotent stem cells (iPSC) and embryonic stem cells (ESC) if undifferentiated cells remain.
- Potential for immune reactions and unintended migration of cells to off-target sites.
- Limited Clinical Evidence
- Most studies remain at phase I/II, with small patient cohorts and short follow-up durations.
- Lack of large-scale randomized controlled trials (RCTs) to confirm efficacy and safety.
- Technical and Cost Barriers
- Cell culture and differentiation require stringent GMP (Good Manufacturing Practice) conditions.
- High production, quality control, and storage costs limit widespread accessibility.
- Ethical and Regulatory Challenges
- Use of ESC faces ethical objections in many countries.
- Need for clear legal frameworks to govern stem cell research and clinical application.
Future Perspectives
- Integration with Biomaterial Technologies: Employing biocompatible scaffolds and hydrogels to enhance stem cell survival, localization, and integration into injured neural tissue.
- Multimodal Therapies: Combining stem cell transplantation with immunomodulatory drugs, growth factors, or electrical stimulation to maximize functional recovery.
- Personalized Medicine: Utilizing autologous iPSCs to reduce graft rejection risk and tailor cell characteristics to individual patients.
- Gene Editing Technologies: Applying CRISPR-Cas9 and other genome-editing tools to improve genetic stability and enhance stem cell differentiation efficiency.
- Predictive Biomarkers for Treatment Response: Developing biomarker panels (including MRI imaging, cerebrospinal fluid analysis, and blood tests) to select suitable patients and monitor post-treatment progress.
- Large-Scale International Clinical Trials: Conducting multi-center, high-quality RCTs with long-term follow-up to provide robust evidence for integrating stem cell therapy into standard care.
References
Kademani, A., Avraam, C., Montenegro, D., Paloh, A., Somannagari, N., Gupta, A., … & Siddiqui, H. F. (2025). Exploring the Emerging Role of Stem Cell Therapy in Neurodegenerative Diseases and Spinal Cord Injury: A Narrative Review. Cureus, 17(8).
Source: Cureus
Link: https://www.cureus.com/articles/379708-exploring-the-emerging-role-of-stem-cell-therapy-in-neurodegenerative-diseases-and-spinal-cord-injury-a-narrative-review#!/authors








