The FASEB Journal, 02/12/2025
The genioglossus is a key therapeutic target in OSAHS. Human umbilical cord mesenchymal stem cells (hUCMSCs), with their regenerative properties and low immunogenicity, offer a promising strategy for its repair.
Introduction
Obstructive Sleep Apnea–Hypopnea Syndrome (OSAHS) is characterized by recurrent collapse of the upper airway during sleep. Airway patency depends primarily on the effective contractile activity of the upper airway dilator muscles. In the pathophysiology of OSAHS, chronic intermittent hypoxia (CIH) plays a key role, causing structural damage and altering the plasticity of the dilator muscles, thereby promoting upper airway obstruction.
The genioglossus muscle is the principal dilator of the upper airway and plays a central role in the pathogenesis of OSAHS. Therefore, therapeutic strategies aimed at enhancing genioglossus muscle function are considered a highly promising approach for improving the clinical management of OSAHS.
urrently, treatment modalities for OSAHS include continuous positive airway pressure (CPAP), mandibular advancement devices (MAD), and hypoglossal nerve stimulation (HNS), along with upper airway muscle training as an adjunctive therapy. However, each modality has certain limitations related to patient adherence, indications, and therapeutic efficacy. Notably, there is still no approved pharmacological therapy that directly improves upper airway muscle function, underscoring the need to develop targeted therapeutic strategies, particularly those focusing on the genioglossus muscle.
Mesenchymal stem cell (MSC) therapy has emerged as a promising research direction in regenerative medicine due to its capacity for self-renewal, multilineage differentiation, and promotion of tissue repair. Among MSC sources, human umbilical cord mesenchymal stem cells (hUC-MSCs) are distinguished by their high differentiation potential, low immunogenicity, abundant availability, and lack of ethical concerns, facilitating clinical application.
Recent studies have shown that MSCs have therapeutic potential in OSAHS by modulating inflammatory responses and restoring tissue damage induced by intermittent hypoxia; additionally, dental pulp stem cells have been demonstrated to protect the genioglossus muscle from hypoxia-induced injury. However, the protective role of hUC-MSCs against genioglossus muscle injury in OSAHS has not yet been clarified. This review aims to synthesize the existing evidence and evaluate the therapeutic potential of hUC-MSCs in mitigating genioglossus muscle injury associated with OSAHS.
The PALM Model
The heterogeneity of Obstructive Sleep Apnea–Hypopnea Syndrome (OSAHS), as conceptualized in the PALM model—including pharyngeal critical closing pressure (Pcrit), arousal threshold, ventilatory control loop gain, and upper airway muscle responsiveness—is reflected in the diversity of risk factors, clinical manifestations, and, in particular, the underlying pathophysiological mechanisms. These differences are often not fully considered in the development of diagnostic tools and treatment strategies. Recent studies emphasize the clinical importance of phenotyping OSAHS within the framework of precision medicine, thereby supporting the implementation of individualized treatment and management strategies tailored to specific phenotypes.
The theoretical framework proposed by Wellman, Eckert, and colleagues identifies four key pathophysiological components contributing to OSAHS, including mechanical upper airway obstruction, upper airway muscle responsiveness, instability of ventilatory control (loop gain), and the arousability of the central nervous system. Identification of these distinct physiological traits enables the development of targeted therapeutic interventions, thereby improving treatment efficacy and optimizing patient outcomes in line with personalized medicine.
The PALM model provides a comprehensive framework for explaining both structural and non-structural factors involved in the onset and progression of OSAHS, including elevated Pcrit, a low arousal threshold, increased loop gain, and impaired function of upper airway dilator muscles, particularly the genioglossus muscle. These factors are closely interrelated, collectively contributing to upper airway collapse during sleep and representing potential therapeutic targets (Figure 1). Notably, injury to the genioglossus muscle may initiate or exacerbate OSAHS by reducing airway patency.
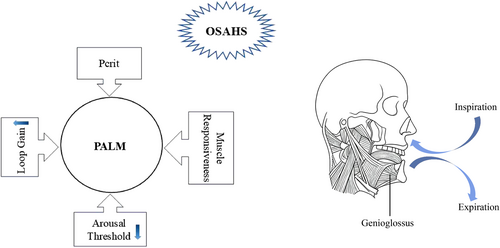
Figure 1. The diagram of PALM model. While the genioglossus, innervated by the hypoglossal nerve, normally contracts during inspiration to stabilize the airway, its compromised function during sleep represents a failure of this neuromuscular compensation. This failure, within a susceptible physiological environment defined by Pcrit, loop gain, and arousal threshold, directly causes or aggravates OSAHS.
A clear understanding of the multifactorial etiology of OSAHS is a critical foundation for guiding the selection of individualized and effective treatment strategies. The absence of approved pharmacological therapies that directly target upper airway muscle function remains a major clinical challenge. Therefore, elucidating the underlying pathogenic mechanisms of OSAHS is of pivotal importance for the identification and development of novel pharmacological therapies.
Effects of OSAHS on Genioglossus
- Oxidative Stress, Muscle Fatigue, and Mitochondrial Energy Metabolism
- Increased oxidative stress in OSAHS:
Patients with OSAHS exhibit elevated levels of reactive oxygen species (ROS) and increased systemic oxidative stress; animal models have also demonstrated enhanced oxidative stress in skeletal muscles, including the genioglossus muscle.
- Muscle fiber composition determines fatigue resistance:
Upper airway muscles have a heterogeneous fiber composition; slow-twitch fibers are fatigue-resistant due to oxidative metabolism, whereas fast-twitch fibers are more prone to fatigue because they rely on glycolysis.
- Alterations in genioglossus muscle fibers in OSAHS:
OSAHS induces progressive changes in the composition and function of genioglossus muscle fibers, leading to structural damage, functional impairment, and exacerbation of airway collapse, thereby creating a pathological vicious cycle of muscle fatigue and respiratory failure.
- Central role of mitochondria:
Mitochondria are the primary energy source of muscle cells; chronic intermittent hypoxia (CIH) causes mitochondrial damage in the genioglossus muscle, resulting in impaired energy-generating function.
- Mitochondrial ROS and respiratory chain dysfunction:
Mitochondrial-derived ROS damage the electron transport chain (ETC), reduce ATP synthesis, and lead to cellular energy deficiency.
- Imbalance between ETC and ATP (adenosine triphosphate):
An imbalance between ETC activity and ATP demand results in ATP depletion, ROS accumulation, mitochondrial swelling, loss of membrane potential, and reduced energy-generating capacity.
- Respiratory chain supercomplexes:
Mitochondria form supercomplexes composed of complexes I–III–IV to enhance electron transfer efficiency, reduce ROS production, and preserve ETC structural integrity.
- Inflammatory Reaction and Apoptosis
- Hypoxia activates oxidative stress–HIF-1α signaling:
Hypoxic conditions increase ROS production, thereby activating and upregulating the expression of hypoxia-inducible factor-1α (HIF-1α).
- Systemic inflammatory response induced by hypoxia:
Hypoxia triggers a systemic inflammatory response accompanied by strong catabolic effects, promoting the degradation of skeletal muscle proteins and functional visceral proteins, and contributing to global metabolic dysregulation.
- HIF-1α and cytokine imbalance:
Nuclear accumulation of HIF-1α increases the secretion of pro-inflammatory cytokines such as TNF-α and IL-6, while suppressing anti-inflammatory cytokines such as IL-10.
- Activation of the NF-κB axis:
The NF-κB signaling pathway is activated under hypoxic conditions, amplifying inflammatory responses through increased expression of pro-inflammatory mediators, including TNF-α, IL-1β, and IL-6.
- Inflammation in the genioglossus muscle during CIH:
CIH increases the expression of TNF-α and IL-6 in the genioglossus muscle, disrupting protein metabolism and promoting structural alterations that lead to muscle injury.
- ROS and mitochondrial damage:
Excessive ROS generated during hypoxia–reoxygenation phases damage cellular membranes and organelles, particularly mitochondria, resulting in reduced mitochondrial membrane potential and impaired mitochondrial function.
- Initiation of apoptosis:
Hypoxic conditions induce oxidative damage and trigger apoptosis in genioglossus myoblasts; animal CIH models demonstrate ultrastructural abnormalities and mitochondrial dysfunction in the genioglossus muscle.
- Functional consequences:
Mitochondrial dysfunction is directly associated with impaired contractile capacity of upper airway muscles, loss of oxidative stress control, and increased apoptotic activity, thereby contributing to the aggravation of OSAHS.
- Research Progress on the Protective Effect of hUCMSCS on Genioglossus in OSAHS
The value of human umbilical cord mesenchymal stem cells (hUC-MSCs) in cell-based research and therapeutic applications has been increasingly recognized. These cells possess several advantageous characteristics, including high proliferative capacity, low immunogenicity, stable expansion under in vitro culture conditions, ease of acquisition from multiple sources, and minimal ethical constraints. In addition, hUC-MSCs exhibit multilineage differentiation potential and are capable of differentiating into cells derived from all three germ layers.
The therapeutic efficacy of hUC-MSCs has been clearly demonstrated in various preclinical disease models, thereby highlighting their potential applications in regenerative medicine and cell-based therapeutic strategies.
- Spotlight on the Homing, Migration and Directional Differentiation of MSCs
- Predominantly paracrine mechanisms of action:
The functional properties of MSCs are mainly attributed to their paracrine activity, through the secretion of soluble bioactive factors and/or the expression of membrane-bound molecules, including adhesion molecules, chemokines, enzymes, growth factors, and interleukins.
- Homing and targeted migration:
The chemotactic homing process of MSCs is regulated by interactions among adhesion molecules such as VCAM-1, PECAM-1, ICAM-1, and CD44, together with hepatocyte growth factor (HGF) and matrix metalloproteinase-2 (MMP-2), enabling the selective recruitment of MSCs to injured tissues.
- Tissue injury signaling:
When tissues are damaged, numerous bioactive molecules are released into the microenvironment as damage-associated signals, playing a critical role in attracting and guiding stem cells to sites of injury.
- Directed differentiation:
Under appropriate inductive conditions, MSCs are capable of differentiating into multiple cell lineages, including osteogenic, chondrogenic, adipogenic, cardiomyogenic, epithelial, hepatic, and neural cells, thereby contributing to tissue repair and restoration of physiological function.
- Application significance of hUC-MSCs:
Owing to the combination of homing capacity, strong paracrine activity, and multilineage differentiation potential, hUC-MSCs have become a central focus of research in regenerative medicine and tissue repair.
- Effects on Skeletal Muscle Regeneration
- Intrinsic regenerative capacity of skeletal muscle:
Skeletal muscle is a highly adaptive tissue and possesses intrinsic regenerative capacity following injury or genetic damage.
- MSCs promote muscle repair and regeneration:
MSCs have been shown to promote the repair and regeneration of both muscle and bone tissues, making them a major focus of research in the treatment of acute and chronic musculoskeletal disorders.
- Myogenic differentiation:
Under appropriate inductive conditions, MSCs can differentiate into muscle cells, contributing to the restoration of muscle fiber structure and contractile function, particularly in the context of muscle injury or degeneration.
- Paracrine role in muscle regeneration:
In addition to direct differentiation, MSCs secrete a variety of bioactive factors such as growth factors, cytokines, and extracellular matrix components, thereby supporting myogenesis and angiogenesis.
- Regulation of the inflammatory microenvironment:
Factors secreted by MSCs help modulate local inflammatory responses, creating a favorable environment for muscle tissue regeneration and repair.
- Paracrine mechanisms
The pivotal role of mesenchymal stem cells (MSCs) in promoting tissue regeneration and repair is primarily mediated through paracrine mechanisms. MSCs secrete a wide range of bioactive molecules, among which sphingosine-1-phosphate (S1P) has been shown to stimulate myoblast proliferation, thereby contributing to skeletal muscle regeneration. In addition to soluble factors, extracellular vesicles (EVs) derived from hUC-MSCs exhibit tissue-protective effects by reducing oxidative stress and inhibiting inflammatory responses, as reported in models of ischemia–reperfusion injury. Notably, exosomes—a subpopulation of EVs secreted by MSCs—play an important role in maintaining tissue homeostasis and promoting skeletal muscle regeneration by enhancing angiogenesis and myogenic processes, thereby supporting the restoration of muscle structure and function.
- Other mechanisms
Mesenchymal stem cells (MSCs) also exert therapeutic effects through their anti-inflammatory and immunomodulatory properties, which are closely associated with their ability to regulate oxidative stress. Although reactive oxygen species (ROS) are physiologically generated during energy metabolism and are necessary for maintaining MSC proliferation and function, excessive ROS production disrupts redox balance and causes cellular damage. In response, MSCs activate antioxidant systems, including enzymes such as superoxide dismutase and catalase, as well as non-enzymatic antioxidants such as glutathione, to neutralize ROS and reduce oxidative stress.
At the same time, excessive ROS can activate redox-sensitive transcription factors such as HIF-1α and NF-κB, thereby promoting inflammatory responses. Notably, stabilization of HIF-1α in MSCs enhances their immunomodulatory activity and the secretion of growth factors.
In addition, MSCs regulate immune responses by promoting the polarization of macrophages from the pro-inflammatory M1 phenotype to the anti-inflammatory M2 phenotype, as well as supporting the transdifferentiation of Th17 cells into regulatory T cells, thereby contributing to the maintenance of immune homeostasis. Collectively, these mechanisms demonstrate the broad therapeutic potential of MSCs in inflammatory and immune-mediated diseases.
Conclusion
hUCMSC-based therapies represents a potential adjunct or alternative to CPAP in the treatment of OSAHS. The pathophysiology of genioglossus injury plays a critical role in the onset and progression of OSAHS. hUCMSCs demonstrate the potential to mitigate genioglossus injury via multiple mechanisms, including anti-inflammatory, antioxidant, and regenerative effects. This emerging therapeutic approach holds considerable promise for OSAHS management. The clinical translation of hUCMSCs still requires further progress in mechanistic elucidation, preclinical efficacy, and early-stage clinical evaluation.
References
- Liu, J. Li, H. Guo, D. Yu, and Y. Liu. (2025). Genioglossus Dysfunction in Obstructive Sleep Apnea Hypopnea Syndrome and the Therapeutic Potential of Human Umbilical Cord Mesenchymal Stem Cells. The FASEB Journal 39, no. 23.
Source: The FASEB Journal
Link: https://faseb.onlinelibrary.wiley.com/action/showCitFormats?doi=10.1096%2Ffj.202502966R








