Frontiers in Immunology, 19/12/2025
Introduction
Inflammation plays a central role in the pathogenesis of many acute and chronic diseases, ranging from autoimmune and degenerative disorders to ischemia–reperfusion tissue injury. Although traditional anti-inflammatory therapies help control symptoms, they often do not address underlying immune dysregulation and carry numerous side effects with prolonged use. Therefore, the demand for novel therapeutic strategies capable of comprehensive and sustainable immune modulation is increasingly urgent.
Umbilical cord-derived mesenchymal stem cells (UC-MSCs) have emerged as a promising candidate due to their strong immunomodulatory capacity, low immunogenicity, and abundant, non-invasive source. UC-MSCs can act on multiple components of the inflammatory response through cell–cell contact and secretion of soluble factors, thereby suppressing excessive inflammatory reactions and promoting tissue repair.
In recent years, an increasing number of preclinical and clinical studies have demonstrated the potential of UC-MSCs in treating inflammation-related diseases. This review focuses on analyzing the mechanisms of inflammation regulation by UC-MSCs, the current clinical evidence, as well as future directions to optimize efficacy and promote the clinical application of this therapy.
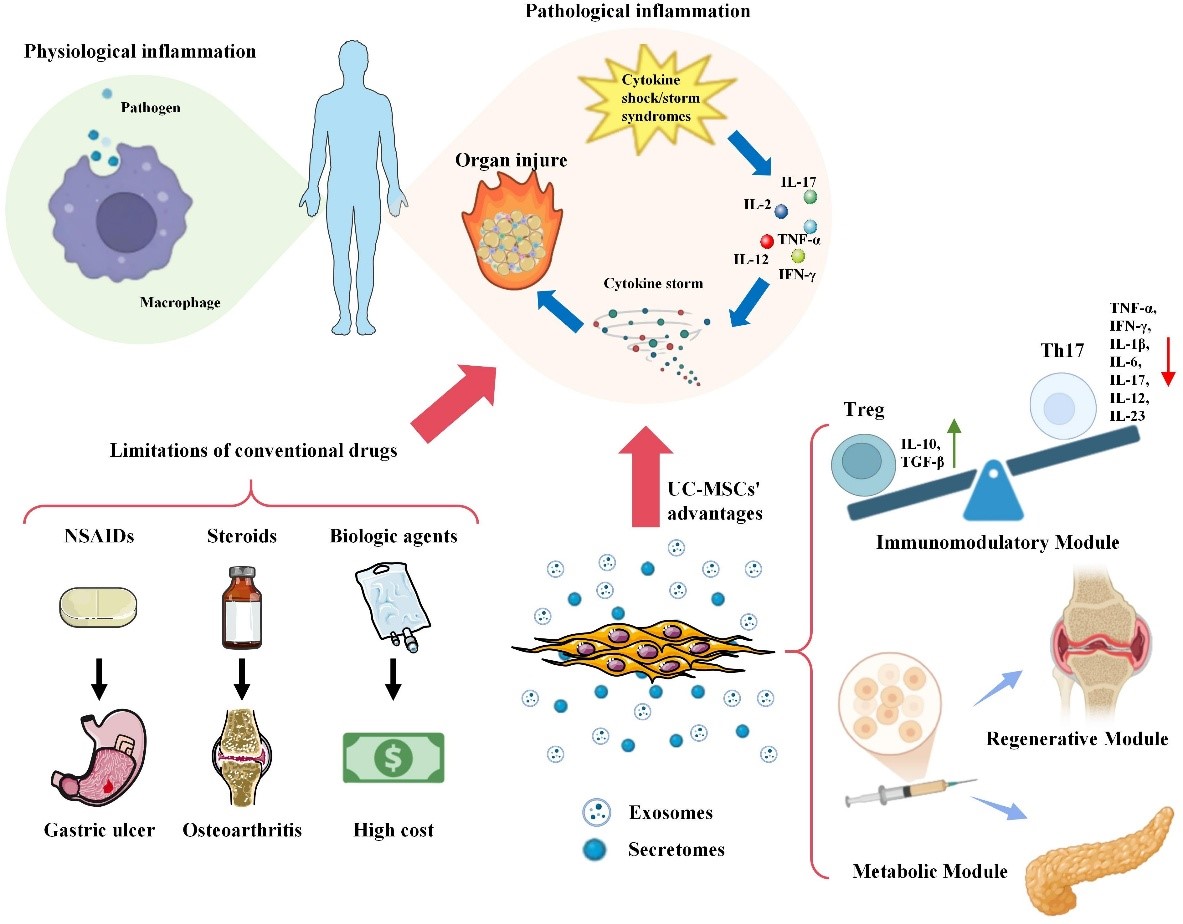
UC-MSCs overcome the limitations of traditional anti-inflammatory therapies through a multi-modal mechanism. Conventional drugs, (such as NSAIDs, steroids, and biologics, are constrained by side effects, cost, and the development of resistance. Human umbilical cord-derived mesenchymal stem cells (UC-MSCs) present a paradigm shift through three core modules.
Modular mechanisms of inflammation regulation by UC-MSCs
The therapeutic efficacy of UC-MSCs in inflammation regulation does not arise from a single molecular pathway but is generated by a highly adaptive, synergistic modular mechanism. Under the influence of inflammatory microenvironmental signals, UC-MSCs can flexibly activate relatively independent yet closely interconnected functional modules, including immune cell reprogramming, inflammasome inhibition, and intercellular communication. This mechanism enables UC-MSCs to perform precise, disease-context-dependent immune modulation while promoting tissue repair and regeneration.
- Immune Cell Reprogramming Module
UC-MSCs regulate both adaptive and innate immunity through an inflammation-context-dependent mechanism. In adaptive immunity, UC-MSCs inhibit T cell activation, promote Treg cells, and restore Th17/Treg balance via IL-10, TGF-β, and signaling–miRNA regulatory axes, contributing to the improvement of autoimmune diseases and GVHD. In innate immunity, UC-MSCs and their exosomes direct macrophage and microglia polarization toward an anti-inflammatory phenotype through metabolic–epigenetic regulation. Due to their flexible response to the inflammatory environment, UC-MSCs function as a dynamic immunometabolic checkpoint, distinct from classical immune checkpoints.
- Inflammasome inhibition module
UC-MSCs suppress inflammasome-dependent inflammatory responses, particularly NLRP3, through multiple coordinated mechanisms. UC-MSCs and their exosomes prevent NLRP3 assembly and activation while regulating macrophage immunometabolism toward an anti-inflammatory profile, reducing pro-inflammatory cytokine secretion. Additionally, UC-MSCs maintain mitochondrial quality control, limit mitoDAMPs release, and inhibit caspase-1 activation, thereby interrupting intracellular inflammatory signaling cascades. Depending on the disease context, UC-MSCs also interact with specific signaling axes to enhance anti-inflammatory and antioxidant effects. Experimental evidence, including 3D culture models and exosome studies, indicates that this module plays a central role in controlling inflammation and tissue degeneration.
- Intercellular communication module
UC-MSCs regulate inflammation and tissue regeneration primarily through intercellular communication, including paracrine signaling and direct contact. Via exosomes, UC-MSCs deliver bioactive molecules that promote tissue repair, extracellular matrix remodeling, and angiogenesis. Additionally, UC-MSCs can directly transfer organelles, particularly mitochondria, to damaged cells through tunneling nanotubes (TNTs), contributing to the restoration of energy metabolism and target cell function. The efficacy of this module can be enhanced by engineering strategies such as 3D culture, the use of biomaterial scaffolds, and exosome purification or design to improve specificity and immunoregulatory efficiency. Preclinical evidence indicates that the intercellular communication module plays a crucial role in ameliorating tissue injury and has significant potential for clinical translation.
Clinical research on UC-MSCs in inflammation-related diseases
Preclinical studies have demonstrated the favorable safety profile and significant therapeutic efficacy of UC-MSCs, and numerous clinical trials are underway to evaluate their potential application in treating inflammatory diseases. The U.S. National Institutes of Health’s ClinicalTrials.gov database records nearly 100 clinical trials worldwide investigating the use of UC-MSCs in inflammatory conditions. Geographic analysis shows that research activity is primarily concentrated in China, the European Union, and the United States. However, 82.2% of the studies remain in phases I and II, while only 5.6% have progressed to phases III and IV. Although early data support the safety of UC-MSCs, key issues such as optimal dosing, routes of administration, and long-term therapeutic efficacy still require systematic and comprehensive evaluation.
- Inflammatory bowel disease
IBD, including ulcerative colitis and Crohn’s disease, is a chronic inflammatory disorder of the gastrointestinal tract. Clinical studies have shown that UC-MSCs are safe and effective in treating IBD via intravenous infusion or local injection. In corticosteroid-dependent Crohn’s disease patients, UC-MSC infusion (4 doses, 1 × 10⁶ cells/kg) significantly reduced disease activity (CDAI, HBI) and decreased corticosteroid requirements compared to the control group, with mild and transient adverse effects. In severe ulcerative colitis, UC-MSCs markedly improved mucosal lesions, lowered Mayo and histological scores, and enhanced quality of life, without serious adverse events reported. Overall, UC-MSCs represent a potential, safe, and effective therapeutic option for IBD patients who are refractory or poorly responsive to standard treatments.
- Cirrhosis
Liver cirrhosis is the consequence of chronic hepatic inflammation and fibrosis caused by various factors. Studies have shown that MSC therapy, particularly UC-MSCs, is safe and effective in treating cirrhosis and progressive liver failure. UC-MSCs help improve liver function, reduce inflammation, modulate immunity (decreasing IL-6, TNF-α; increasing IL-10, Treg), lower the incidence of liver failure, and enhance survival, with very few serious adverse effects.
- COVID-19
Severe COVID-19 is associated with excessive inflammatory responses and cytokine storms. Multiple clinical trials have shown that UC-MSCs are safe and effective in treating severe COVID-19/ARDS, helping reduce inflammation (IL-6, TNF-α, CRP), improve pulmonary oxygenation, decrease lung damage on CT, and increase survival rates. UC-MSCs also support long-term restoration of lung structure, with no serious adverse events reported. However, further studies are needed to optimize the timing of intervention, dosage, and treatment protocols.
- Arthritis
Osteoarthritis (OA) is a chronic disease with no currently available long-term effective treatment. UC-MSCs, due to their proliferative, migratory, and immunoregulatory abilities, have the potential to promote cartilage regeneration. A phase I/II clinical trial showed that intra-articular injection of UC-MSCs (particularly with a repeated injection regimen) was safe and significantly improved pain and joint function compared to hyaluronic acid over 12 months of follow-up, with no serious adverse effects observed, although no clear differences were seen on MRI.
- Graft-versus-host disease (GVHD)
GVHD is a severe complication following allogeneic hematopoietic stem cell transplantation, with an aGVHD incidence of approximately 40% and a significant impact on long-term prognosis. UC-MSCs demonstrate notable efficacy in the treatment and prevention of GVHD due to their strong immunoregulatory capacity. Clinical studies show that UC-MSCs achieve a response rate of 59–63% in steroid-refractory aGVHD, with better outcomes in children and mild GVHD, while also improving survival. In prevention, UC-MSCs significantly reduce the incidence of aGVHD and cGVHD without increasing the risk of leukemia relapse. The main mechanisms include restoring immune balance, mobilizing MDSCs, and regulating inflammation via exosome/miRNA. Overall, UC-MSCs have been shown to be safe and provide multidimensional benefits in GVHD management.
- Lupus
Systemic lupus erythematosus (SLE) is the most common and severe form of lupus, causing multi-organ damage and being difficult to treat. UC-MSCs have demonstrated significant immunoregulatory efficacy in SLE, particularly through inhibition of B cells (CD19⁺) and modulation of T cell activation, with superior effects compared to exosomes alone. Long-term follow-up studies confirm safety and sustained efficacy, with a 5-year survival rate of 84% and 34% of patients achieving remission, accompanied by improvements in disease activity indices and serological markers. These data support UC-MSCs as a potential therapeutic approach for refractory SLE.
- Rheumatoid arthritis
Rheumatoid arthritis is a chronic inflammatory disease with a high risk of disability, in which UC-MSCs demonstrate strong immunoregulatory effects by inhibiting T, B, DC, and NK cells, thereby reducing inflammatory responses. Phase I/II clinical trials in refractory RA patients have shown that UC-MSCs are safe long-term (follow-up 1–3 years) and significantly reduce inflammatory markers (ESR, CRP, RF, anti-CCP) while improving joint function (DAS28, HAQ), demonstrating their potential therapeutic efficacy.
- Ankylosing spondylitis
Ankylosing spondylitis is a chronic inflammatory disease causing spinal pain, stiffness, and limited mobility, associated with immune dysregulation (reduced Treg). Preliminary clinical studies show that intravenous UC-MSCs are safe, causing only transient mild fever, and reduce disease activity while improving function (decreased BASDAI, BASFI, pain; lowered ESR, CRP, TNF-α, ICAM). Meta-analysis indicates overall efficacy higher than conventional treatment. Additionally, UC-MSCs combined with HA scaffolds support bone formation and enhance quality of life in vertebral lesions. However, larger-scale studies are needed to confirm efficacy.
- Allergic rhinitis
Allergic rhinitis is a common respiratory disease caused by IgE-mediated type I hypersensitivity, characterized by Th1/Th2 immune imbalance, increased Th2 response, mast cells, and eosinophils. UC-MSCs, due to their immunoregulatory capacity and low immunogenicity, can migrate to the nasal mucosa, helping restore Th1/Th2 balance and increase Treg cells, thereby improving symptoms. An early-phase clinical trial (NCT05151133) is currently evaluating the feasibility, safety, and dosing of intravenous UC-MSCs for AR treatment, providing a basis for future clinical studies.
Clinical studies indicate that UC-MSCs demonstrate consistent efficacy and safety across multiple inflammatory diseases. The most common adverse effect is transient fever, which resolves spontaneously; no serious complications or long-term sequelae have been reported. UC-MSCs significantly improve disease activity indices (CDAI in Crohn’s, SLEDAI in lupus, DAS28 in RA) and enhance survival in severe conditions such as COVID-19 and GVHD. Repeated infusion regimens (2–4 doses) generally provide more sustained effects, especially in chronic and severe diseases (GVHD, cirrhosis). Both intravenous and local administration are well tolerated, suitable for systemic and localized conditions. Overall, UC-MSCs exhibit multifunctionality and a modular immunoregulatory–tissue repair mechanism across a broad spectrum of diseases.
Clinical efficacy assessment and treatment plan optimization
Due to the rich biological properties and broad clinical application potential of UC-MSCs, related clinical studies are continuously being conducted. Numerous pieces of evidence indicate that the administration route and dosage of UC-MSCs are key factors directly influencing therapeutic efficacy. Based on existing clinical research literature, this review summarizes the commonly used cell delivery routes and UC-MSC infusion dosages, aiming to provide a foundation for evaluating treatment efficacy and optimizing regimens in clinical practice.
- Correlation analysis between functional module activity and clinical efficacy
The therapeutic efficacy of UC-MSCs in inflammatory diseases is closely dependent on the activity of their functional modules, with anti-inflammatory cytokine secretion, particularly IL-10, serving as a key indicator. Numerous analyses show that IL-10 levels strongly correlate with clinical improvement (e.g., reduction in Mayo score in inflammatory bowel disease), confirming the central role of the immunoregulatory module.
In addition, dosage and administration frequency are decisive factors for treatment efficacy. Intravenous infusion regimens with varying doses and schedules generally demonstrate significant clinical improvement and high safety, with transient mild fever being the most common adverse effect. Other delivery routes, such as intra-articular injection (20×10⁶ cells in osteoarthritis) or intrathecal injection (1×10⁶ cells/kg weekly × 4 doses), also show symptomatic improvement, with adverse effects mostly mild and temporary.
Overall, clinical data indicate a clear dose–response relationship, highlighting that a deep understanding of the link between UC-MSC functional module activity (lesion-targeting, immunoregulation, tissue repair) and clinical efficacy is essential for developing safe, effective, and personalized treatment strategies.
- Pharmacokinetic modeling of viable cells and optimizing treatment protocols
Optimizing UC-MSC therapy requires developing dosing strategies based on a living cell pharmacokinetic model, comprising three phases: administration – distribution – elimination. This model helps predict the temporal kinetics of UC-MSC activity, thereby determining the optimal route, dose, frequency, and infusion interval.
- Clinical evidence:
- GVHD prevention: dose ~1×10⁶ cells/kg per week achieves sustained immunoregulatory effects.
- Spinal cord injury (intrathecal injection): multiple doses (1×10⁶ cells/kg/week × 4) provide significantly higher efficacy than a single dose, with mild and transient adverse effects.
→ Confirms the need to maintain cell activity through repeated administration.
- Optimization of dosing interval and treatment duration: reduces the risk of immune responses while enhancing efficacy; supports personalized regimens.
- Biomarker-based dosing algorithm: proposed relationship Dose = k × ln(IL-6) + C, reflecting diminishing benefits at high IL-6 levels; UC-MSCs have been shown to reduce IL-6, thereby improving therapeutic response.
- Dose–response:
- High doses provide stronger immunoregulatory effects (healthy volunteers).
- Cirrhosis (hepatic artery injection): 4×10⁸ cells, 2 doses reduce liver failure, with good safety.
Optimal regimens should integrate UC-MSC pharmacokinetics + inflammatory biomarkers (e.g., IL-6) + individual patient characteristics. Ongoing trials will continue refining precise dosing strategies for inflammatory diseases.
Future directions and emerging paradigms
Although UC-MSCs hold significant potential for treating inflammatory diseases, clinical application still faces many challenges, requiring a focus on cell optimization and personalized therapy.
- Enhancement of UC-MSCs: Genetic engineering (CRISPRa) to upregulate IL-10, PD-L1; 3D culture and bioreactor “training” of cells in an inflammatory environment to improve post-infusion efficacy.
- UC-MSC Exosomes (MSC-Exos): A next-generation strategy that is safe, minimally immunogenic, with controllable pharmacokinetics and easy standardization. They contain miRNA, cytokines, and functional proteins, achieving efficacy comparable to MSCs while reducing tumorigenic and graft rejection risks; can be engineered for targeted delivery and on-demand drug release.
- Precision medicine based on biomarkers: Predicting responses using IL-6, TNF-α, Th17/Treg ratios, and machine learning; standardizing cell quality based on functional outputs (IL-10, exosomes) rather than surface markers.
- Standardization and long-term management: Global standards covering donor selection, culture, storage, and cell recovery; optimizing timing, route, and individualized dosing; requiring long-term monitoring systems and regulatory frameworks for UC-MSCs and MSC-Exos.
UC-MSCs and their derivatives represent a multifunctional, modular therapeutic model. Future success depends on three transitions: from natural → engineered, from empirical → precision medicine, and from exploratory research → standardized practice.
References
Yin, Li & Sun, Chen-yang & Chen, Gui-lai & Xiang, Zhuo & Hu, Bao-quan & Zhou, Fang & Wang, Qiang. (2025). Modular mastery of inflammation: umbilical cord mesenchymal stem cells as a therapeutic frontier. Frontiers in Immunology. 16. 10.3389/fimmu.2025.1721947.
Source: Frontiers in Immunology
Link: https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2025.1721947/full