Old muscle stem cells express high levels of the cancer-associated molecule CD47. Blocking a pathway mediated by CD47 restored strength to old mice in a Stanford Medicine study.
Stanford Medicine, November 15, 2022
Muscle stem cells, the cells in muscle fibers that generate new muscle cells after injury or exercise, lose their potency with age. But a study by researchers at Stanford Medicine shows that old mice regain the leg muscle strength of younger animals after receiving an antibody treatment that targets a pathway mediated by a molecule called CD47.
The findings are surprising because CD47, billed as the “don’t eat me” molecule, is better known as a target for cancer immunotherapy than for muscle regeneration. It peppers the surface of many cancer cells, protecting them from immune cells that patrol the body to root out and engulf dysfunctional or abnormal cells. Now it seems old muscle stem cells may use a similar approach to avoid being culled by the immune system.
There are important clinical implications as well. If the results in mice hold true in humans, it may one day be possible to boost muscle recovery after surgery and fend off the decline in muscle strength that occurs with aging or after periods of inactivity.
“Rejuvenating the muscle stem cell population in older mice led to a significant increase in strength,” said Helen Blau, PhD, professor of microbiology and immunology. “This is a localized treatment that could be useful in many clinical settings, although more work needs to be done to determine whether this approach will be safe and effective in humans.”
Blau, the Donald E. and Delia B. Baxter Foundation Professor and director of the Baxter Laboratory for Stem Cell Biology, is the senior author of the study, which was published online Nov. 15 in Cell Stem Cell. Former instructor Ermelinda Porpiglia, PhD, is the lead author.
Pioneering technique
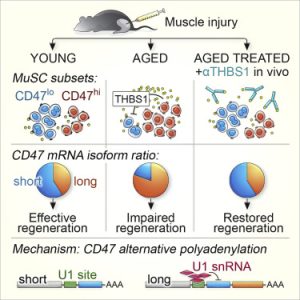
It’s well known that muscle stem cells lose their ability to divide rapidly in response to injury or exercise as they age. But it’s not clear exactly why — it’s been difficult to tease apart the various subpopulations of the cells for study. Porpiglia used a technique pioneered at Stanford Medicine called single-cell mass cytometry to identify subpopulations of mouse muscle stem cells, the surface markers of which change in relative proportion as animals age.
One molecule stood out when she used the technique: CD47. The molecule was found at high levels on the surface of some muscle stem cells, but at lower levels on others. In younger animals, the low-level cells predominate. But the balance shifted dramatically when she looked at muscle stem cells from older animals. These mice had many more high-level cells than younger animals.
“This finding was unexpected because we primarily think of CD47 as an immune regulator,” Porpiglia said. “But it makes sense that, much like cancer cells, aged stem cells might be using CD47 to escape the immune system.”
Porpiglia found that high levels of CD47 on the surface of muscle stem cells correlate with a decrease in their function. These cells were less able to engraft, or establish themselves, and make new muscle when transplanted into the leg muscles of other mice, compared with cells with lower levels of the molecule.
Further investigation revealed that a molecule called thrombospondin, which binds to CD47 on the surface of the muscle stem cells, suppresses the muscle stem cells’ activity. Thrombospondin accumulates in skeletal and cardiac muscle with age, but its expression in skeletal muscle decreases after exercise.
Porpiglia showed that an antibody that recognizes thrombospondin and blocks its ability to bind to CD47dramatically affected the function of muscle stem cells: Cells isolated from older animals divided more robustly when growing in a laboratory dish in the presence of the antibody, and when the antibody was injected into the leg muscles of old mice the animals developed bigger and stronger leg muscles than control animals. When given prior to injury, the antibody helped the aged animals recover in ways similar to younger mice — again generating larger and stronger muscles than old animals who had received a placebo treatment.
The anti-thrombospondin antibody had no effect in mice genetically engineered to be unable to make CD47, indicating that the interaction of CD47 with thrombospondin is critical to the activity of the muscle stem cells in response to aging and injury.
Potential treatment to rebuild muscle
“Anti-thrombospondin antibody treatment is a localized and transient way to boost muscle stem cell activation in these animals,” Porpiglia said. “We are hopeful that it might one day be possible to inject an antibody to thrombospondin at specific sites in the body to regenerate muscle in older people or to counteract functional problems due to disease or surgery.”
The study points to the role played by CD47 in several biological processes. The researchers plan to investigate whether antibodies that block CD47’s don’t-eat-me signal will stimulate the immune system to remove old, dysfunctional muscle stem cells.
“It will be interesting to study whether cancer immunotherapies that target CD47 also block the thrombospondin pathway,” Blau said. “If so, it is possible that anti-CD47 antibodies could counteract some of the muscle wasting, known as cachexia, that commonly occurs in cancer patients.”
The study was funded by the National Institutes of Health (grants NS089533 and AG020961), the California Institute for Regenerative Medicine, the Baxter Foundation and a BD Biosciences Stem Cell grant.
Nguồn: https://www.sciencedirect.com/science/article/abs/pii/S1934590922004271
Reference:
Ermelinda Porpiglia, Thach Mai, Peggy Kraft, Colin A. Holbrook, Antoinede Morree, Veronica D. Gonzaleze, Keren I. Hilgendorf, Laure Frésard, Angelica Trejo, Sriram Bhimaraju, Peter K. Jackson, Wendy J. Fantl, Helen M. Blau. Elevated CD47 is a hallmark of dysfunctional aged muscle stem cells that can be targeted to augment regeneration. Cell Stem Cell, 2022; 29(12) DOI: 10.1016/j.stem.2022.10.009
Source: Stanford Medicine
Link: https://med.stanford.edu/news/all-news/2022/11/leg-strength-old-muscles.html