Parent’s Guide to Cord Blood, September , 2024
We are continuing our tradition of issuing periodic updates on trends in clinical trials that employ perinatal sources of stem cells. This update extends our timeline through the end of 2023.
| Cell Source | Cumulative # Trials Advanced Cell Therapy Through end of 2023 |
| Umbilical Cord Blood +/- Non-Perinatal Sources | 316 |
| Umbilical Cord Tissue +/- Non-Perinatal Sources | 581 |
| Other Perinatal Sources (Placenta, Amniotic) & Mixed Perinatal Sources | 149 |
Below is a timeline of the number of clinical trials per year that performed advanced cell therapy with cells sourced from umbilical cord blood, umbilical cord tissue, or other/mixed perinatal sources. This graph is compatible with our last perinatal trial review through the end of 2021 and can be viewed as an extension of that timeline1.
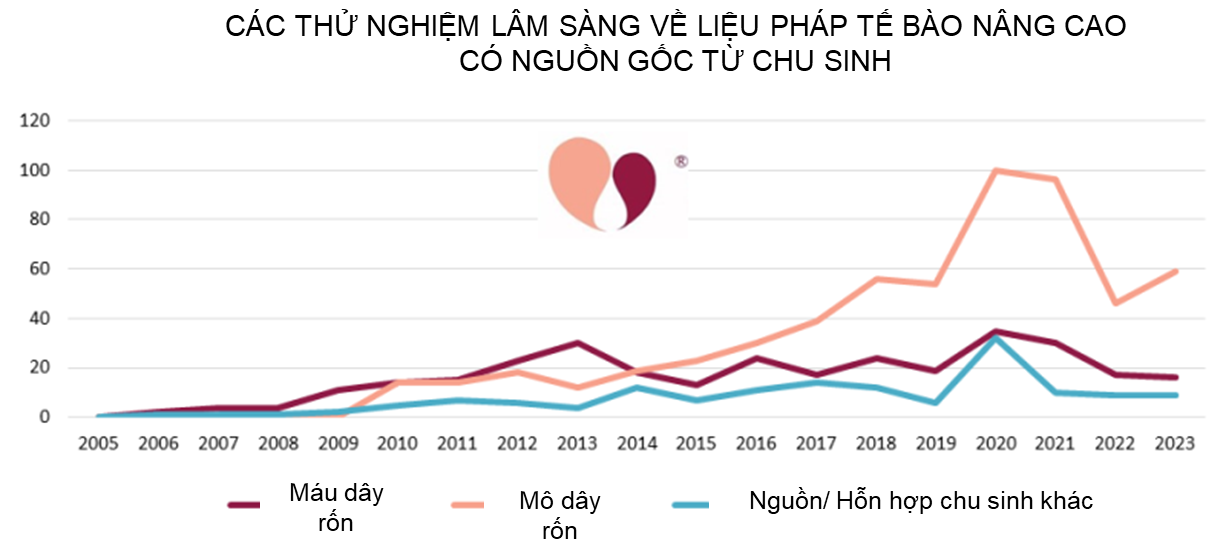
Some readers may be shocked that the number of clinical trials employing umbilical cord mesenchymal stromal cells (UC-MSC) has dropped by almost half since a peak in 2020-2021. In fact, this is not surprising, and it reflects the impact of the COVID-19 pandemic on the cell therapy field. Our team at CellTrials.org has previously documented that the COVID-19 pandemic caused a 16% surge in overall registrations of advanced cell therapy clinical trials2. But for MSC specifically, the number of clinical trial registrations doubled from 2019 to 20202. We have also published a report noting that during 2020-2022, 41% of the advanced cell therapy trials for COVID-19 relied on perinatal sources of cells3. By April 2023, 65% of the published outcomes from these trials were studies that employed cells of perinatal origin3. And finally, the largest meta-analysis of MSC therapies for COVID-19 found that infusions of MSC yielded improved patient survival (RR=0.63 with 95% Confidence Interval 0.46 to 0.85)4. Despite the big community effort that went into perinatal clinical trials for COVID-19, it should not be surprising that with the pandemic winding down the yearly number of advanced cell therapy trials from perinatal sources has returned to pre-pandemic levels. Nonetheless, over the two years since our last summary, from the end of 2021 to the end of 2023, the cumulative number of perinatal trials in advanced cell therapy increased by 17% from 891 to 1046.
We have also created pie charts of the diagnoses treated in the 538 perinatal advanced cell therapy trials registered over the five years 2019-2023. This time period was chosen to showcase recently registered trials, and to bracket the COVID-19 era. In previous reviews we have tried to group trials purely on the basis of cell source, into cord blood trials versus cord tissue trials. But this has always been overly simplistic, because cord blood can be a source of immunotherapy cells or it can be used to culture MSC. And there is also the question of how to include trials sourced from the placenta. The placenta holds blood, the blood vessels have endothelial cells, the placental tissue can be used to culture MSC, and the amniotic membrane holds epithelial cells. In this year’s review, we have decided to create three groups of perinatal cell therapy trials based on the cellular mechanism of action: immunotherapy, MSC, and everything else.
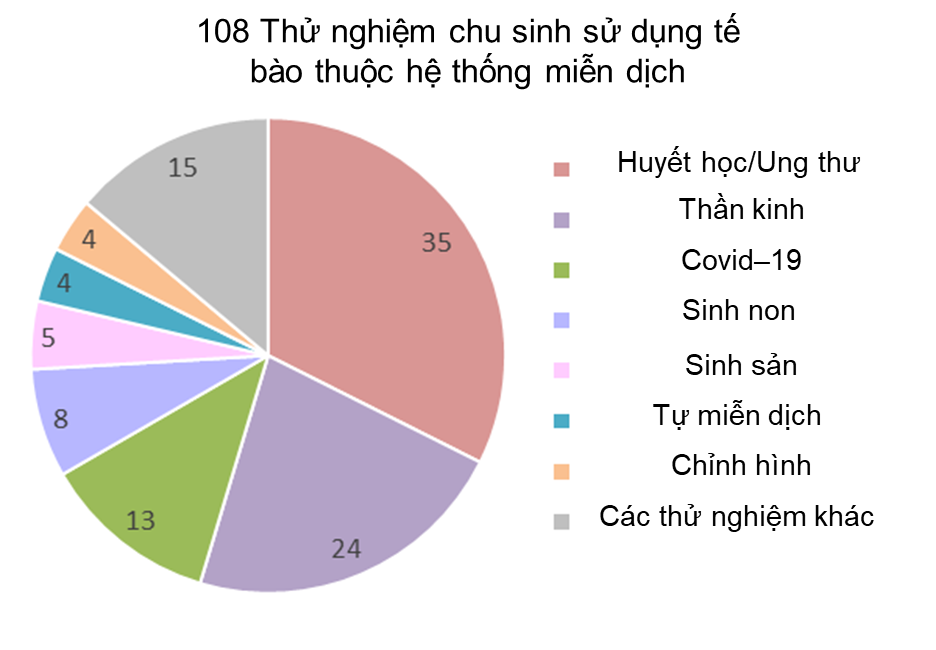
Our first pie chart shows the diagnosis categories for 108 perinatal trials registered 2019-2023 which employ cells that belong to the immune system. These include unmanipulated MNC from cord blood or placenta blood, and immunotherapy cells cultured from cord blood or placental blood. Only 6% (6/108) of the trials use placental blood, but 40% (43/108) of the trials are deriving specific immunotherapy cell types from perinatal blood. The manufactured cell types include: expanded CD34+, NK, CAR-NK, TCR-NK, CIK, MAK, CAR-T, and T-reg cells. The largest diagnosis group for the years 2019-2023 is Hematology/Oncology, holding 32% (35/108) of the trials. It is notable that 86% (30/35) of this group is composed of immunotherapy trials that employ cells which have been expanded or derived from cord or placental blood. The remaining 14% (5/35) of Hematology/Oncology trials which employ unmanipulated cord blood as part of advanced cell therapy are all located in Asian countries. Note that the Neurologic diagnosis group, which represents 22% (24/108) of the cord blood trials, includes neurologic complications associated with birth, such as Hypoxic Ischemic Encephalopathy and Cerebral Palsy, as well as stroke and adult neurodegenerative disorders. The separate diagnosis category Premature Birth is dominated, at 63% (5/8), by trials for bronchopulmonary dysplasia, and includes other premature birth complications which are not neurologic.
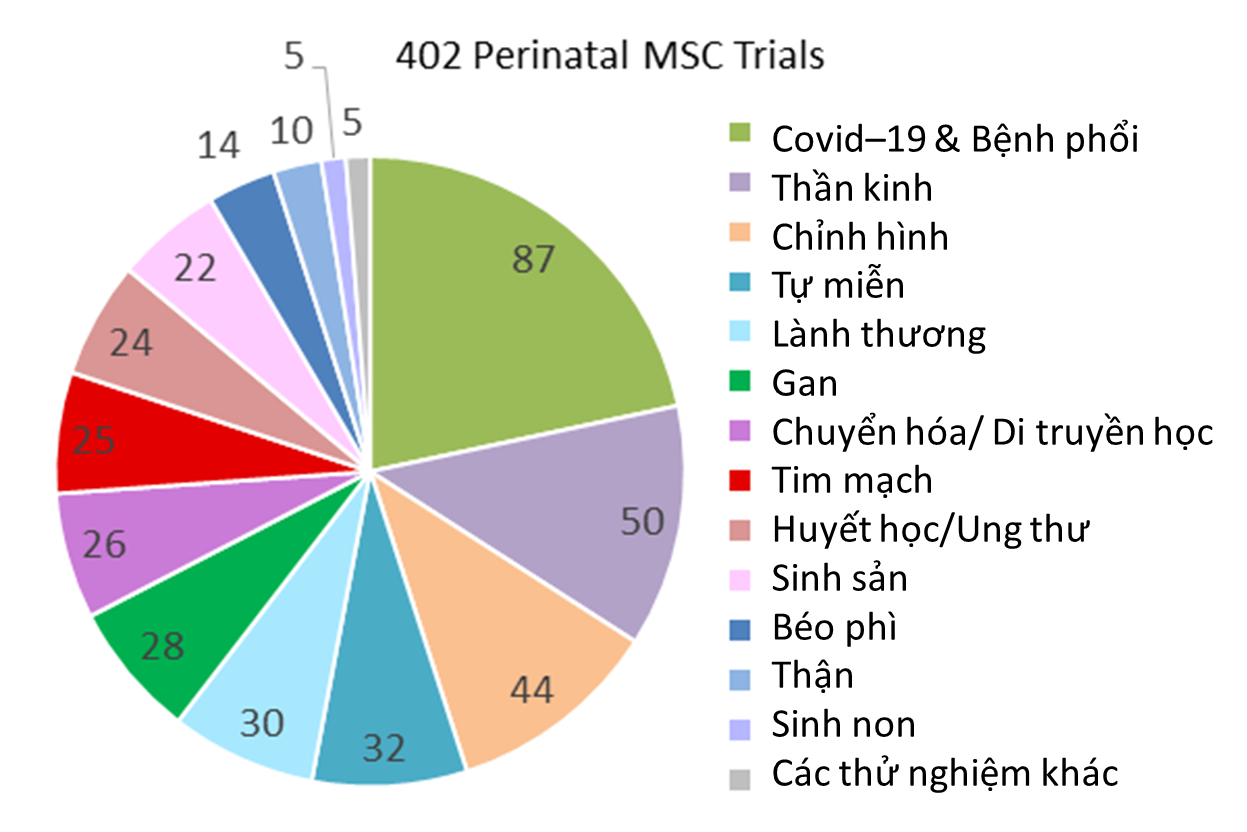
Our second pie chart shows the diagnosis categories for 402 perinatal trials registered 2019-2023 which employ MSC. Cord blood was the starting material for deriving MSC in 4% (17/402) of these trials, with 53% (9/17) of those trials located in South Korea. Also 9% (36/402) of the trials derived MSC from placenta or amniotic membrane or multiple perinatal sources. The majority of the trials, 87% (349/402), derived MSC only from umbilical cord tissue, although the MSC may have been delivered together with other (non-perinatal) cells or agents or seeded on a scaffold. When sorted by diagnosis category, the largest indication for use was COVID-19 and other lung conditions, at 22% (87/402), not surprisingly. Once again, the second largest category of trials is Neurologic conditions, holding 12% (50/402) of the MSC trials, and Orthopedic trials are third with 11% (44/402) of the MSC trials. The most noticeable aspect of the pie chart of perinatal MSC trials is that they are fairly evenly distributed among multiple medical indications, with seven diagnosis categories each holding about 7% of the perinatal MSC trials on average.
Among the 402 clinical trials with perinatal MSC registered 2019-2023, only 1.2% (5/402) were designed to compare the clinical efficacy of perinatal MSC versus adult sources of MSC. There were slightly more trials, 1.5% (6/402), that tested UC-MSC against its conditioned medium (which effectively holds exosomes from UC-MSC). Much more work is needed to clarify the clinical impact of the biological differences between perinatal MSC and adult MSC that have been identified in laboratory studies5.
Finally, there is a group of 28 perinatal trials registered 2019-2023 which do not employ immunotherapy cells or MSC. In this catch-all category, 50% (14/28) of trials employ amniotic epithelial cells, 21% (6/28) employ sheets of amniotic membrane as scaffolding for other cells, and 18% (5/28) employ endothelial cells from umbilical cord blood vessels.
This year’s update on clinical trials of perinatal advanced cell therapy demonstrates that research in this field is ongoing at the same pace as before the COVID-19 pandemic, employs a variety of cell types, and is targeting multiple indications for use.
References
- Verter F. 2022 Update: How many clinical trials use cord blood or cord tissue? Parent’s Guide to Cord Blood Foundation Newsletter Published 2022-08
- Verter F, Silva Couto P, Bersenev A. New trials of cell-based therapy surged in 2020, both because of and despite the pandemic. org News Published 2021-04-25
- Verter F, Silva Couto P. 2023 Update: Development of COVID-19 Therapies from Birthing Tissues and Cord Blood. SCTM 2023; 12S1:S8.
- Couto PS, Al-Arawe N, Filgueiras IS, Fonseca DLM, Hinterseher I, Catar RA, Chinnadurai R, Bersenev A, Cabral-Marques O, Moll G, Verter F. Systematic review and meta-analysis of cell therapy for COVID-19: global clinical trial landscape, published safety/efficacy outcomes, cell product manufacturing and clinical delivery. Frontiers Immunology 2023; 14:1200180.
- Silva Couto P, Stibbs DJ, Rotondi MC, Khalife R, Wolf D, Takeuchi Y, Rafiq QA. Biological differences between adult and perinatal human mesenchymal stromal cells and their impact on the manufacturing processes. Cytotherapy 2024; vol:1-13.
Source: Parent’s Guide to Cord Blood
Link: https://parentsguidecordblood.org/en/news/2024-update-how-many-clinical-trials-employ-perinatal-sources-stem-cells