The Scientist, September 13 , 2024
Two decades ago, Caroline Gargett identified adult stem cells in the endometrium. Now, she explores their functions to improve women’s health.

Adult stem cells have captured researchers’ interest since their discovery in the 1960s. Due to their unique capacity to self-renew and differentiate into multiple cell types, scientists find them promising for treating health conditions such as cancer and neurological diseases.
By the mid-2000s, researchers identified small populations of adult stem cells in many human organs. Yet, the existence of these cells in the endometrium puzzled Caroline Gargett, a biologist at the Hudson Institute of Medical Research who has studied this tissue for nearly three decades. “When I got to hear about the endometrium and that three quarters of it sheds each month, and then it re-epithelializes and grows, I thought ‘there had to be stem cells in this tissue,’” she said.
Innately curious about how things work, Gargett looked for these cells in the tissue that lines the uterus. Gradually, Gargett and her team not only discovered stem cells in the human endometrium, but also characterized and started exploring the endometrial stem cells’ potential applications in improving women’s health.1
Building a Path to the Endometrium
The endometrium is a complex multicellular, bilayer tissue that lines the uterus. Each month, in the absence of pregnancy, its outermost layer sheds and regrows, creating a cycle of proliferation, differentiation, and breakdown that repeats approximately 400 times in a woman’s life without losing function or scarring.2
Gargett’s research journey into the endometrium’s biology started in the late 1990s when she landed a position as a postdoctoral researcher in the laboratory of Peter Rogers at Monash University to work on reproductive biology. In her initial studies, she focused on the mechanisms regulating endometrial angiogenesis. Physiological angiogenesis in women is a frequent process that takes place in the ovaries and endometrium as part of the dynamic changes that occur in these tissues during the menstrual cycle.3 Gargett and her colleagues showed that the levels of vascular endothelial growth factor (VEGF), a key regulator of angiogenesis during development, did not correlate with endothelial cell proliferation, a well-established marker of angiogenesis, in endometrium biopsy samples from healthy women.4 While looking at microvascular endothelial cells (MECs), which are involved in the creation of new vessels from existing ones, the team found that the two estrogen receptors had different expression patterns.5 In a subsequent study exploring the effects of sex steroids on angiogenesis, they also showed that exposure to 17beta-estradiol, the most potent, naturally-occurring estrogen, but not progesterone stimulated the expression of VEGF and its cognate receptor in MECs, revealing hormonal regulatory elements that influence this process in the endometrium.6
While investigating the formation of vessels in the uterus lining, another idea brewed in Gargett’s mind: the existence of stem cells in the endometrium. During her graduate studies in the laboratory of hematologist James Wiley at the Austin Hospital in Melbourne, Gargett was exposed to the potential applications of stem cells as the clinical staff at the hospital was developing approaches to harvest hematopoietic stem cells for transplantation into women who had received higher doses of chemotherapy and needed to rescue their bone marrow after treatment.7 This experience sparked Gargett’s fascination with the topic, which continued even as she moved away from the hematology field and started studying the endometrium.
In Search of Endometrial Stem Cells
Since the late 1970s, researchers proposed that the human endometrium may contain adult stem cells.8,9 These undifferentiated cells can turn into some, but not all, cell types, in contrast to the pluripotent nature of embryonic stem cells. Adult stem cells play a role in the maintenance and repair of the tissue in which they reside, attributes that are even more critical in tissues with high cellular turnover, such as the skin, intestines, bone marrow, and endometrium.
Despite these early hypotheses about the likely presence of adult stem cells in the uterus lining, by the early 2000s, there was no experimental evidence supporting that idea, which puzzled Gargett. “I couldn’t really understand why no one had really looked at it,” she said. “So, I was determined to do something about it.”
Gargett suspected that these endometrial stem cells resided in the basalis, the innermost layer of the endometrium that helps regenerate the layer that sheds every month during menstruation. Therefore, she needed tissue samples that contained both endometrium layers. In the early 2000s, hysterectomies, the surgical removal of the uterus, were a common procedure in Australia, and Gargett was fortunate to have access to these wombs that would otherwise be discarded.
To search for adult stem cells in the endometrium, Gargett drew inspiration from the approaches researchers had used to look for these cells in other tissues. Traditionally, scientists identified adult stem cells by assessing their clonogenic activity, which is the ability of a single cell to form a colony.
At that time, Gargett and two graduate students that she supervised set up the clonogenic assays they needed to identify these cells. After obtaining the endometrial samples, they purified single-cell suspensions of epithelial and stromal cells that were cultured in very low amounts. They found that a small number of these cells formed colonies and that a similar set of growth factors supported their proliferation, providing the first experimental evidence for the existence of a stem-cell-like population in the endometrium.1
Defining the Cells
Endometrial stem cells could provide another source of multipotent adult stem cells for cell-based therapies. Therefore, an important next step for Gargett as she established her own independent research team was to identify specific markers that would enable their isolation.
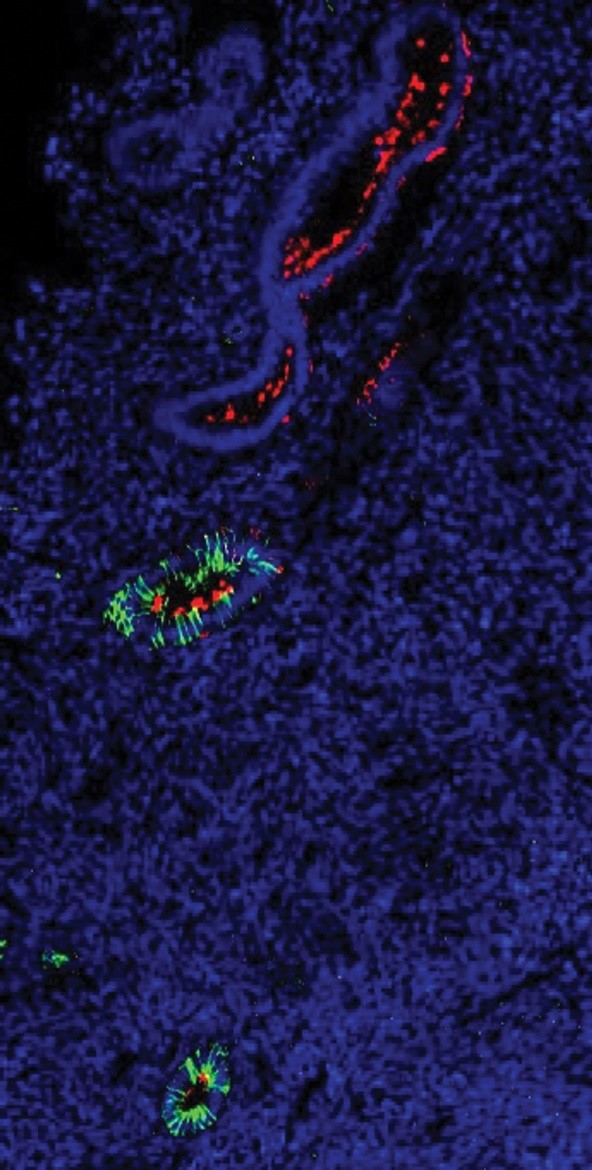
In the deepest layer of the endometrium, endometrial stem cells (red) are found close to glands (green).
Initial studies in endometrial stem cells revealed that these cells expressed markers—such as CD146, a member of the immunoglobulin superfamily, and platelet-derived growth factor-receptor β (PDGF-Rβ)—which were previously used to identify stem cells in other tissues.10,11 Additionally, in collaboration with Hans-Jörg Bühring, a stem cell researcher at the University of Tubingen, Gargett’s team found that they could robustly enrich their endometrial stem cell pool by using the monoclonal antibody W5C5.12
These markers allowed the researchers to show that endometrial stem cells localized near blood vessels and, when stimulated with specific factors, differentiated into adipogenic, chondrogenic, osteogenic, and myogenic cell lineages, demonstrating that these cells exhibited the multipotency characteristic of adult stem cells.11-13
Although the existence of stem cells in the endometrium was anticipated due to the highly regenerative nature of the tissue, the discovery of these cells “added another layer of important contribution to the field of stem cell research,” said Reshef Tal, a physician scientist and reproductive endocrinologist at Yale University, who believes that Gargett’s work has provided important insights into endometrial regeneration and repair. Moreover, the identification and further characterization of these cells enabled Gargett’s group and others to start exploring their involvement in pathological conditions that affect the female reproductive tract as well as their potential application for the treatment of diseases that interfere with women’s health.
A Story of Sheep and Endometrial Stem Cells
While Gargett initially focused on characterizing and understanding the role of endometrial stem cells on the tissue’s biology, she soon realized that these cells could also help treat conditions such as pelvic organ prolapse (POP) in women. POP occurs when the muscles and connective tissue of the pelvic floor weaken, causing one or more pelvic organs to prolapse from their normal position. These muscles and tissues may weaken or tear due to pregnancy, childbirth, or age, but other biological factors also increase the risk for POP.14
While women with less severe stages of this condition can manage it with non-surgical strategies, severe cases of POP often require reconstructive surgery that is frequently combined with surgical meshes. However, high rates of adverse events led the US Food and Drug Administration (FDA) to issue warnings that raised concerns about the safety of this treatment.14
With one in four women affected by POP, Gargett felt compelled to find new treatment alternatives for this condition, and she thought that endometrial stem cells might help. Developing surgical meshes with more degradable materials, which could then be combined with endometrial stem cells, was a project that piqued the interest of Shayanti Mukherjee, a bioengineer at the Hudson Institute of Medical Research. “I was from an engineering background, and she was in reproductive biology. We wanted to combine our forces for women’s health,” said Mukherjee.
Mukherjee met Gargett when she joined Gargett’s laboratory in the mid-2010s as a postdoctoral researcher. At that time, Gargett was already testing tissue engineering approaches to tackle POP. In an initial study, the researchers developed a gelatin-coated polyamide mesh that was either seeded or not with labeled human endometrial mesenchymal stem cells (eMSCs).15 In a rat model of wound repair, the team found that meshes with cells had more neovascularization and less infiltration of immune cells compared to meshes with no eMSCs, suggesting that these cells promoted wound repair and reduced the inflammatory reaction toward the mesh.
While the rodent model provided some evidence of a beneficial effect of eMSC-coated meshes, the skin repair model did not recapitulate the changes seen in women with POP. Previous studies suggested that ewes develop spontaneous POP.16 Because human and ovine vaginal tissues are similar, Gargett decided to study female sheep and found that those who had given birth more than once (multiparous) had thinner and weaker vaginal wall muscles, similar to women who had given birth, suggesting that these animals may be a good model for studying female POP.17
“Generally, with preclinical models, there is an intervention that you need to do to create the disease,” explained Mukherjee. “In this one, we don’t have to create a model; we just have to screen and find out who has [POP].”
Using these multiparous ewes, Gargett, Mukherjee, and their colleagues then tested whether a mesh seeded with ovine eMSCs would restore the strength of the prolapsed vaginal musculature.18 When comparing the gelatin-coated and non-gelatin-coated meshes, regardless of whether ovine eMSCs were seeded, the team found poor tissue integration. To circumvent that issue, Mukherjee developed a hydrogel to deliver the eMSCs, and the meshes were implanted following a two-step procedure where the researchers first inserted the construct and then applied the hydrogel. Using this approach, the team saw that the ovine eMSCs lasted for 30 days and that the constructs provoked less inflammation and disruption of the vaginal musculature.
By developing tissue engineering approaches using eMSCs, Mukherjee hopes to overcome the shortcomings of previous strategies and provide women affected by POP with more treatment options. “There’s magic in our uterus. We want to take that magic out and give it back to women, so they can heal from all the pelvic floor disorders that happen due to childbirth and have long term, dire consequences on their lives,” said Mukherjee.
Helping to Fight Diseases and Improve Diagnosis
Beyond POP treatment, endometrial stem cells may also assist in the diagnosis and treatment of other gynecological conditions.
According to Tal, endometrial stem cells could provide a less invasive source of adult stem cells because they could be easily obtained from the menstrual fluid or endometrial biopsies. In terms of future applications, Tal emphasized that these cells could be used for a variety of reproductive conditions, including implantation failure, recurrent pregnancy loss, and uterine scarring, such as that seen in patients with Asherman’s syndrome. Women with this syndrome have little to no endometrium, which makes it difficult for them to have children, Gargett noted. She suggested that endometrial stem cells could be harvested from small amounts of the patient’s endometrium and used to seed a decellularized tissue that could be then implanted back into the patient to grow the uterus lining.
A better understanding of how endometrial stem cells contribute to endometriosis could also help develop new treatments and diagnostics for this chronic disorder in which the endometrial tissue escapes from the uterus and forms lesions that cause debilitating pain and infertility.19 In a previous study, Gargett’s team identified endometrial stem cells in the peritoneal and menstrual fluids of women with and without endometriosis, but found no differences in the concentrations of these cells.20 While these findings support the cells’ involvement in the etiology of endometriosis, they also suggest that they may not be useful for diagnosis. As Gargett is interested in using menstrual fluid as a noninvasive diagnostic tool for this disorder, her team is looking for other factors that might serve this purpose. “If we could find something that was quite specific, or a panel of markers that was specific, it could mean that girls could have menstrual fluid analyzed early on, rather than waiting for them to be old enough to have a laparoscopy or even the imaging,” explained Gargett.
While Gargett and others have explored some of the functions of endometrial stem cells, Tal remains curious about the role these cells play during pregnancy and postpartum uterus remodeling. Additionally, he believes that a better characterization of the different stem cell subtypes and their potential biological roles would also be important because evidence suggests that these cells may either originate locally or migrate to the uterus from other locations, such as the bone marrow.21
“Caroline provided a really very good basis for what we are doing now,” noted Alexander Nikitin, a stem cell and cancer researcher at Cornell University who has followed Gargett’s work over the years. “We’re really developing new methods, which are really benefiting very much from her initial studies.”
According to Gargett, while two decades of studies have shed some light on endometrial stem cell functions, there is still much more to explore in the highly dynamic endometrium. “I kind of call it the Cinderella tissue in the stem cell world because it’s been overlooked and unrecognized,” Gargett said. “It’s fascinating and it’s worth studying because I think there’s lessons to be learned.”
References
- Chan RW, et l. Clonogenicity of human endometrial epithelial and stromal cells. Biol Reprod. 2004;70(6):1738-1750.
- Critchley HOD, et al. Physiology of the endometrium and regulation of menstruation. Physiol Rev. 2020;100(3):1149-1179.
- Gargett CE, Rogers PA. Human endometrial angiogenesis. Reproduction. 2001;121(2):181-186.
- Gargett CE, et al. Lack of correlation between vascular endothelial growth factor production and endothelial cell proliferation in the human endometrium. Hum Reprod. 1999;14(8):2080-2088.
- Gargett CE, et al. Estrogen receptor-alpha and -beta expression in microvascular endothelial cells and smooth muscle cells of myometrium and leiomyoma. Mol Hum Reprod. 2002;8(8):770-775.
- Gargett CE, et al. 17Beta-estradiol up-regulates vascular endothelial growth factor receptor-2 expression in human myometrial microvascular endothelial cells: Role of estrogen receptor-alpha and -beta. J Clin Endocrinol Metab. 2002;87(9):4341-4349.
- Gu BJ, et al. James Saville Wiley (1936–2022): pioneer of purinergic research in Australia and abroad. Purinergic Signal. 2023;19(4):581-585.
- Prianishnikov VA. On the concept of stem cell and a model of functional-morphological structure of the endometrium. Contraception. 1978;18(3):213-223.
- Padykula HA. Regeneration in the primate uterus: the role of stem cells. Ann N Y Acad Sci. 1991;622:47-56.
- Schwab KE, et al. Identification of surface markers for prospective isolation of human endometrial stromal colony-forming cells. Hum Reprod. 2008;23(4):934-943.
- Schwab KE, Gargett CE. Co-expression of two perivascular cell markers isolates mesenchymal stem-like cells from human endometrium. Hum Reprod. 2007;22(11):2903-2911.
- Masuda H, et al. A novel marker of human endometrial mesenchymal stem-like cells. Cell Transplant. 2012;21(10):2201-2214.
- Gargett CE, et al. Isolation and culture of epithelial progenitors and mesenchymal stem cells from human endometrium. Biol Reprod. 2009;80(6):1136-1145.
- Food and Drug Administration. Pelvic organ prolapse (POP). 2019.
- Ulrich D, et al. Human endometrial mesenchymal stem cells modulate the tissue response and mechanical behavior of polyamide mesh implants for pelvic organ prolapse repair. Tissue Eng Part A. 2014;20(3-4):785-798.
- Couri BM, et al. Animal models of female pelvic organ prolapse: lessons learned. Expert Rev Obstet Gynecol. 2012;7(3):249-260.
- Emmerson S, et al. Ovine multiparity is associated with diminished vaginal muscularis, increased elastic fibres and vaginal wall weakness: implication for pelvic organ prolapse. Sci Rep. 2017;7:45709.
- Emmerson S, et al. Composite mesh design for delivery of autologous mesenchymal stem cells influences mesh integration, exposure and biocompatibility in an ovine model of pelvic organ prolapse. Biomaterials. 2019;225:119495.
- Cousins FL, et al. New concepts on the etiology of endometriosis. J Obstet Gynaecol Res. 2023;49(4):1090-1105.
- Masuda H, et al. Endometrial stem/progenitor cells in menstrual blood and peritoneal fluid of women with and without endometriosis. Reprod Biomed Online. 2021;43(1):3-13.
- Abuwala N, Tal R. Endometrial stem cells: origin, biological function, and therapeutic applications for reproductive disorders. Curr Opin Obstet Gynecol. 2021;33(3):232-240.
Source: The Scientist
Link: https://www.the-scientist.com/an-endometrial-stem-cell-pioneer-72146