European Journal of Gastroenterology & Hepatology, 2018
Bin Chen, Yong-Hong Wang, Jin-Qin Qian, et al
Introduction
Acute-on-chronic liver failure (ACLF) occurs in patients with chronic liver disease, which is characterized by acute hepatic injury such as jaundice and coagulopathy [1]. The mortality at 3 months for these patients is mainly because of multisystem organ failure and severe infection [2–4]. In Asia, ACLF is mainly caused by hepatitis B virus (HBV) infections and its mortality is as high as 63–72.3% [5,6].
Currently, liver transplantation is the only therapy that has been proven to be beneficial. However, the rapid disease progression and lack of donors limit its application in patients with HBV-ACLF and a recent Belgium cohort study reported that only 7% of ACLF patients received the therapy of liver transplantation [7]. Therefore, new strategies for these patients are urgently required.
Mesenchymal stem cells (MSC) are multipotent cells that have the potential to differentiate into various types of cells, including hepatocytes [8,9]. Studies have shown that the MSC treatment can ameliorate liver fibrosis [10,11] and reverse fulminant hepatic failure in experimental mice [12]. In 2012, Shi et al. [13] showed that umbilical cordderived MSC (UC-MSC) transplantation can improve the liver function and increase the survival rate, without increased treatment-associated side effects. Also, they suggested that UC-MSC treatment is safe in the clinic and may serve as a novel therapeutic approach for HBVassociated ACLF patients. The present review and metaanalysis aim to systematically assess the efficacy and safety of MSC treatment in patients with HBV-associated ACLF.
Materials and methods
Search strategy and study selection
We performed a systematic literature search of three databases: PubMed (which includes MEDLINE) and Embase.com. The bibliographies of relevant review articles and all included studies were reviewed manually to identify relevant studies. No restrictions were applied to language or publication date because of the limited number of manuscripts.
Inclusion and exclusion criteria
All records identified through database searches were downloaded and duplicate records were removed. The title and abstract of the remaining records were screened for relevance to liver disease and human participants. After this initial screening, the lists of selected studies were crosschecked to resolve discrepancies. Subsequently, full articles were retrieved for a detailed assessment.
Eligibility criteria were as follows: (a) the publications were clinical studies, including randomized-controlled trials, prospective nonrandomized-controlled trials, and case–control studies; (b) the participants were diagnosed with ACLF associated with HBV infection; (c) the participants had received MSC transplantation; (d) the publication language was not limited; (e) the sources of the MSC were not restricted, and the routes of MSC transplantation were not restricted; and (f) if the data overlapped or were duplicated among two or more studies by the same study team, only the study with more complete data or one earlier study were included.
Study selection and data extraction
Two reviewers (B.C. and J.Q.Q.) worked independently to determine whether a study fulfilled the inclusion criteria to assess the methodological validity of each candidate study, and extracted data from those included studies. The reviewers resolved discrepancies by discussion. If no consensus was reached, a third reviewer (Y.H.W.), unaware of previous determinations, functioned as an arbiter.
The following primary items were extracted: first author, publication year, country, study design, sources of MSC, numbers of MSC, routes of MSC transplantation, length of follow-up, demographic data (age and sex), laboratory data before and after stem cell therapy [e.g. levels of albumin (ALB), total bilirubin (TBIL), prothrombin time, alanine aminotransferase (ALT), HBV DNA, aspartate aminotransferase, serum creatinine, and serum α-fetoprotein, model for end-stage liver disease (MELD) score, Child–Pugh score], adverse events, and survival.
Quality assessment
The Newcastle–Ottawa scale was used to assess the quality of nonrandomized studies, which measures quality in three domains, including selection, comparability, and exposure. High-quality studies were considered to have a score of seven or greater. The Cochrane Risk of Bias Tool was used to assess the quality of randomized-controlled trials. Study quality was assessed independently by two investigators (B.C. and J.Q.Q.) and any discrepancies were addressed by a joint discussion of the original article.
Statistical analysis
The primary outcome of this study was the mortality rate at 12 weeks and at the final follow-up. Continuous data, including the levels the MELD score, ALT, ALB, and TBIL, were evaluated by a standardized mean difference (SMD) with a 95% confidence interval (CI). Then, the SMD of each study was combined to yield a pooled SMD. Dichotomous data, including death, and adverse events were evaluated by a risk ratio (RR) with a 95%CI. The RR of each study was then combined to yield a pooled RR. A P value of less than 0.05 was considered statistically significant for the effect size. The heterogeneity among the studies was assessed by the I2 statistic (I2>50% indicated substantial heterogeneity) and the χ 2 -test (P < 0.10 was considered to represent significant statistical heterogeneity). All analyses were carried out using the statistical package Review Manager version 5.3 (The Nordic Cochrane Center, The Cochrane Collaboration, Copenhagen, Denmark).
Result
Study selection and study characteristics
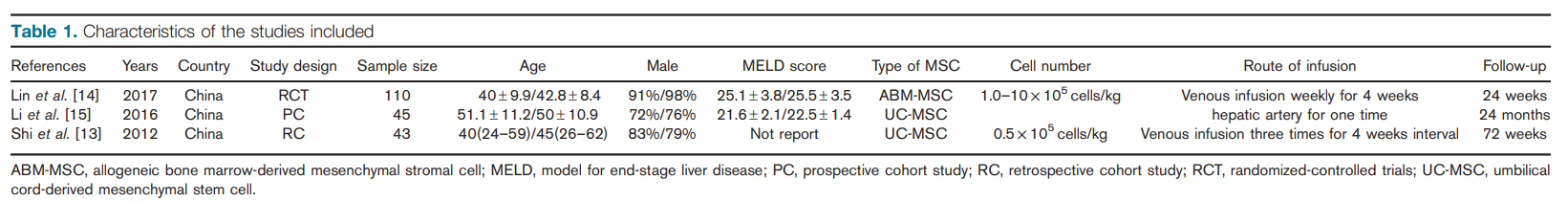
Overall, 753 papers were identified from the three databases; after screening the title and abstract, 12 papers were further evaluated. A total of nine papers were excluded for other reasons as shown in Fig. 1. Finally, three papers were eligible for the meta-analysis [13–15], including one randomized-controlled trial [14], one prospective nonrandomized cohort [15], and one case–control cohort [13]. All the three studies had been carried out in China. A total of 198 ACLF patients were enrolled for this review; 91 patients had been treated with MSC and 107 patients had been treated with standard medical therapy (SMT) as controls. The characteristics of each study are shown in Table 1. According to the methodological assessment, only this randomized-controlled trial was considered to be of high quality and the other two studies were considered to be of low quality.
In 2012, Shi et al. [13] published the results of a case–control study that evaluated the safety and efficacy of UC-MSC transfusion in ACLF patients associated with HBV infection. No significant side effects were observed in the UC-MSC treatment group, and UC-MSC treatment reduced the MELD scores. Serum TBIL and ALT levels were also significantly decreased after the UC-MSC treatment. Furthermore, this study found that the UC-MSC treatment significantly increased the survival rates in ACLF patients. These results suggested that the UC-MSC treatments could aid the recovery of ACLF patients.
In 2016, another prospective study was published by Li et al. [15] that also assessed the efficacy and safety of UCMSC transplantation in HBV-ACLF patients treated with plasma exchange and entecavir. A total of 45 consecutive HBV-ACLF patients were studied prospectively. It was found that patients with transplantation of UC-MSC had a significantly higher cumulative survival rate at 24 months (54.5 vs. 26.5%, P=0.015). Levels of ALB, prothrombin time, and INR in patients with transplantation of UC-MSC were also markedly improved at 24 months (P < 0.05). Also, no severe adverse event was observed in any patient.
Recently, the results of a randomized-controlled trial conducted by a Chinese team were published. A total of 110 HBV-ACLF patients were enrolled in this open-label randomized-controlled trial. The control group (n=54) was treated only with SMT and the experimental group (n=56) was infused weekly for 4 weeks with 1.0∼10×105 cells/kg allogeneic bone marrow-derived MSC. It was found that patients with transplantation of MSC had a significantly higher cumulative survival rate at week 24 (73.2 vs. 55.6%, P=0.03). Compared with the control group, allogeneic bone marrow-derived MSC treatment markedly improved clinical laboratory measurements, including serum TBIL and MELD. It is noteworthy that the incidence of severe infection in the MSC group was much lower than that in the SMT group (16.1 vs. 33.3%, P=0.04).
Meta-analysis of mortality rate
The overall survival rate was reported in all three included studies [13–15]. However, the follow-up duration was different. The follow-up period was 24 weeks in Lin’s study, 24 months in Li’s study, and 72 weeks in Shi’s study.
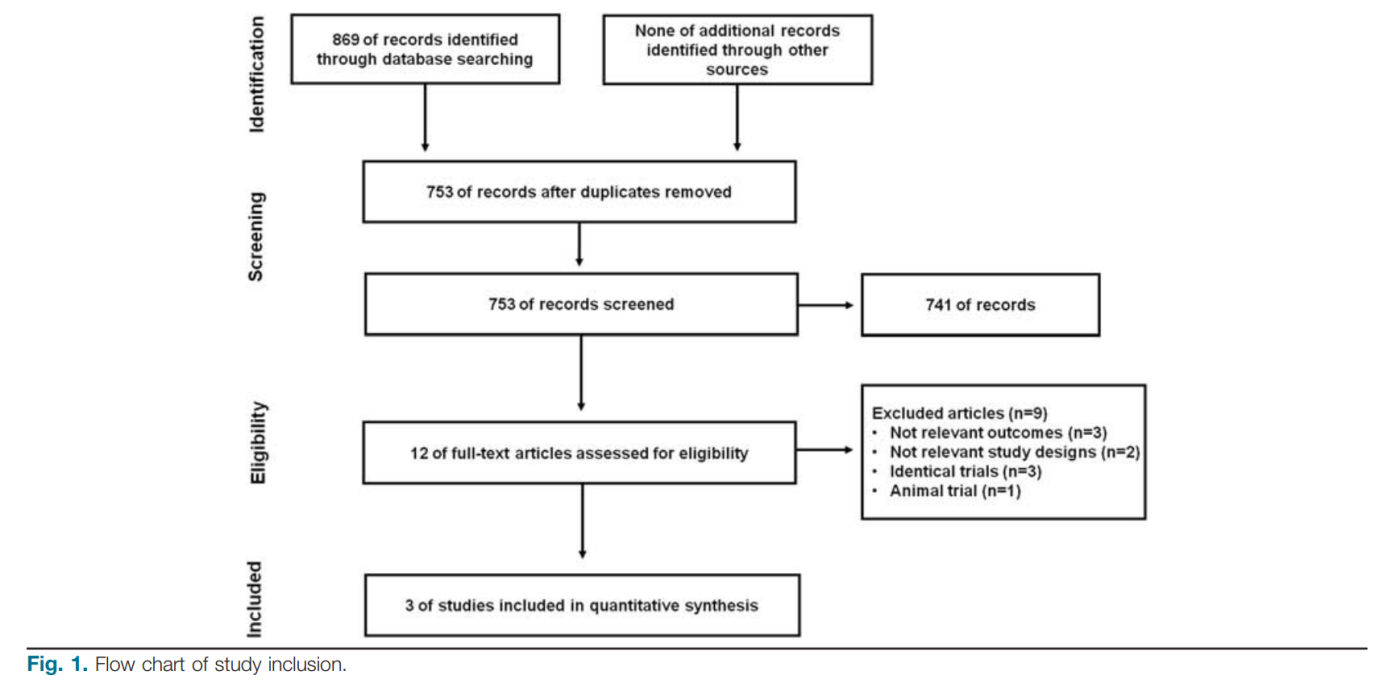
In Shi’s study, five (20.8%, 5/24) patients in the UC-MSC treatment group and 11 (47.4%, 11/19) patients in control group died during the first 12 weeks of follow-up. In Li’s study, 29 patients died within 12 weeks after the treatments, including five (45.4%, 5/11) in the UC-MSC treatment group and 24 (70.5%, 24/34) in the control group. At month 24, 31 patients had died. Of the 31 deaths, five (45.5%) patients were in the UC-MSC treatment group and 26 (76.5%, 26/34) patients were in the control group. In Lin’s study, 32 patients died within 12 weeks after the treatments, 11 (19.6%, 11/56) in the MSC treatment group, and 21 (38.3%, 21/54) in the control group. At week 24, 39 patients died, including 15 (26.7%, 15/56) in the MSC treatment group and 24 (48.6%, 24/54) in the control group.
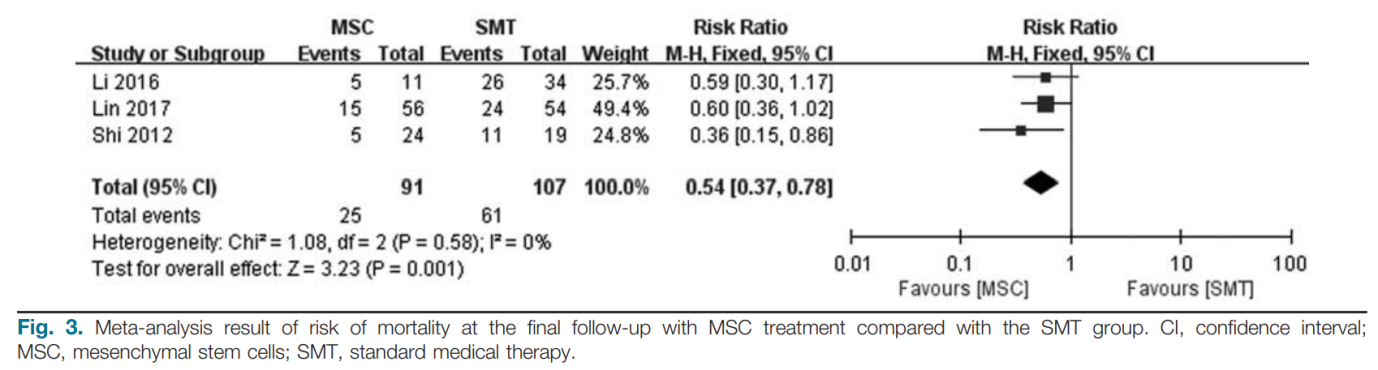
The mortality rate at week 12 after treatment was obtained from all three included studies. In total, 21 (23.1%, 21/91) patients died at week 12 in the MSC group and 56 (52.3%, 56/107) patients died in the control group. Pooled results showed that MSC treatment could significantly reduce the mortality rate at week 12 (RR: 0.50; 95%CI: 0.33, 0.76; P= 0.00009; Fig. 2) compared with SMT. No significant heterogeneity was detected between studies (Q =0.58; I2=0%). Moreover, the mortality rate at the final follow-up after treatment was extracted from all three included studies. In total, 25 (27.5%, 25/91) patients died at 12 weeks in the MSC group and 61 (57%, 61/107) patients died in the control group. Pooled results suggested that MSC treatment could also significantly reduce the mortality rate at the final follow-up (RR: 0.54; 95%CI: 0.37, 0.78; P= 0.001; Fig. 3) compared with the SMT group. No significant heterogeneity was detected between studies (Q =0.58; I2=0%).
Meta-analysis of model for end-stage liver disease score change at 2 weeks after treatment
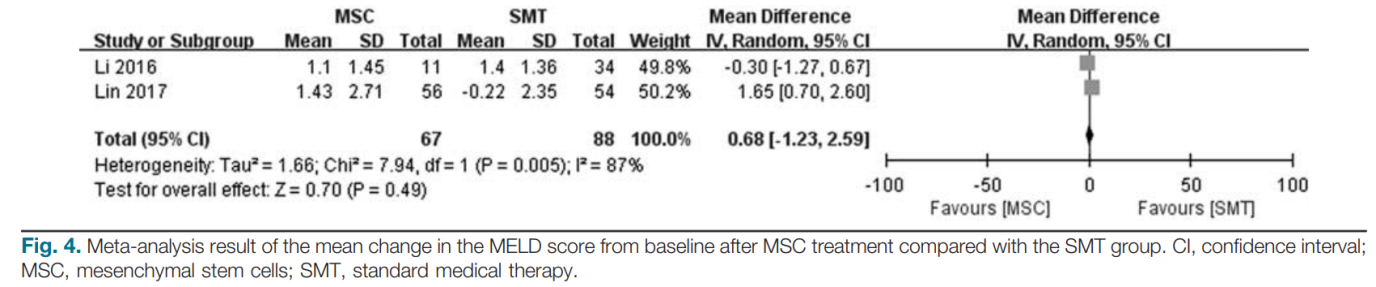
Two studies reported the changes in MELD scores from baseline [14,15]. Lin’s study found that the MELD score in the MSC group had decreased more dramatically than that in the SMT group at week 2. However, MSC treatment did not lead to a more significant reduction in the MELD score at week 2 compared with the SMT group in Li’s study. Pooled estimates showed that MSC treatment did not lead to a more significant reduction in the MELD score at week 2 for ACLF patients (MD: 0.68; 95%CI: − 1.23, 2.59; P=0.49; Fig. 4) compared with the SMT group. Significant heterogeneity was detected between studies (Q = 0.005; I2=87%). Therefore, a random-effects model was applied for the meta-analysis.
Meta-analysis of changes in liver function outcomes after treatment
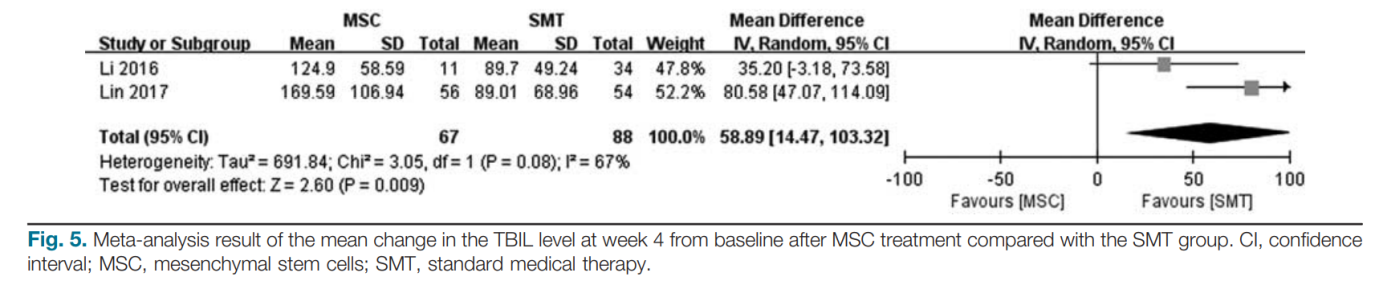
Two studies reported the changes in ALT, TBIL, ALB, and INR [14,15]. Pooled estimates showed that MSC treatment did not lead to a more significant reduction in ALT (MD: 29.99; 95%CI: − 89.61, 149.59; P=0.62), TBIL (MD: 39.28; 95%CI: − 61.76, 140.32; P=0.45), ALB (MD: 0.06; 95%CI: − 1.00, 1.11; P=0.92), and INR (MD: 0.01; 95% CI: − 0.08, 0.1; P=0.84) at week 2 for ACLF patients compared with the SMT group. However, pooled estimates showed that MSC treatment could significantly reduce the value of TBIL at week 4 (MD: 58.89; 95%CI: 14.47, 103.32; P=0.009; Fig. 5) compared with the SMT group. A random-effects model was applied for these meta-analyses.
Adverse effects associated with mesenchymal stem cells treatment
In Shi’s study [13], two patients developed a self-limiting fever (body temperature: 37–38°C) within 2–6 h after the UC-MSC transfusions, but recovered within 12 h without any additional treatment. In the control group, none of the patients developed a fever. No complications or side effects were found in the UC-MSC-treated patients during the follow-up period. Also, no severe side effects or complications (including hepatalgia, hemorrhage, fever, etc.) were observed after the procedure in Li’s study [15]. Lin’s study observed that the incidence of infection in the MSC group was much lower than that in the SMT group (25.0 vs. 44.4%; P=0.03) [14], but no differences were observed in incidences of other severe complications (e.g. encephalopathy, hepatorenal syndrome, gastrointestinal bleeding) between the SMT and the MSC groups.
Discussion
Our present systematic review and meta-analysis aimed to evaluate the efficacy and safety profiles of MSC treatment in patients with ACLF associated with HBV infection. The results suggested that MSC treatment could significantly reduce the mortality rate, without increasing the incidence of severe complications. However, there is controversy in terms of the benefit of MSC treatment in the improvement of liver function and the MELD score.
For one new therapy, the safety profiles were widely concerned by clinicians and patients. Three studies reported this outcome, and none of the studies observed severe adverse events associated with the transfusion of MSC in patients with ACLF compared with the SMT group. However, the incidence rate of fever was significantly higher in the MSC group than in the SMT group. It is noteworthy that the fever was limited, with no need for a medical intervention. Therefore, the present evidence suggested that MSC treatment was safe for patients with HBV-ACLF.
Another important question is the impact of MSC treatment on the survival of patients with HBV-ACLF. For patients with ACLF, it is important to determine whether patients can survive for the first 3 months. Kjaergard et al. [16] reported a mortality rate of 51% in their systemic meta-analysis. The short-term mortality may be as high as 65% at month 3. Obviously, MSC treatment could help ACLF patients survive from the first 3 months of diseases. Our pooled results showed that MSC treatment could significantly reduce the mortality rate at week 12 and at the final follow-up. Moreover, according to Lin’s results, this benefit might be associated with the reduction in the incidence of severe infection in the MSC group compared with the SMT group (16.1 vs. 33.3%, P=0.04). Furthermore, mortality from multiple organ failure and severe infection was higher in the SMT group than in the MSC group (37.0 vs. 17.9%; P= 0.02). Immune imbalance and systemic inflammation play important roles in the development of liver failure. Lin’s results suggested that MSC treatment might serve immunomodulation and anti-inflammation functions, which could alleviate the hepatic inflammation, improve liver function, decrease the incidence of severe infection, and thus improve the survival rate. Shi’s study found that the level of α-fetoprotein increased from 14 ng/ml at the baseline to 7170 ng/ml at week 1 after the first UC-MSC treatment, and it remained at a high level during the subsequent 8 weeks of follow-up, which was not observed in the control group. This meant that UC-MSC transfusions may promote hepatocyte proliferation, and thus help patients recover from ACLF, especially for the first 3 months.
The effect of MSC treatment on the liver function and the MELD score is controversial. Although the MSC Table 1. treatment could significantly improve the liver function and the MELD score compared with the baseline in the MSC group, our pooled results suggested that MSC treatment could not markedly improve the MELD score, ALT, ALB, and INR compared with the control group.
Another issue is the regimen of MSC treatment, including the frequency of MSC treatment, the route of administration of MSC, the source of MSC, and the cell number of MSC. In Shi’s study, UC-MSC therapy was administered three times at 4-week intervals with ~ 0.5 × 106 cells/kg of body weight, a single transplantation of UC-MSC was administered of about 100 × 106 cells through the proper hepatic artery in Li’s study, and allogeneic bone marrow-derived MSC was infused weekly for 4 weeks with 1.0–10 × 105 cells/kg. This suggested that MSC treatment was effective through the route of both the vein and the hepatic artery. Future trials could evaluate the relative efficacy of the different routes, explore the optimal number of stem cells, and assess the optimal frequency of MSC treatment.
The strength of our present systematic review and meta analysis was limited by the number and quality of the studies included. Only one RCT was included, and the other two studies were nonrandomized control trials, which might have increased the bias in the study.
Furthermore, the sample size of the study was relatively small, especially for these two nonrandomized-controlled studies. Another limitation was that all studies had been carried out in China, and no relevant data were published by researchers from other countries. Therefore, the conclusion from our review cannot be generalized to other countries. In addition, publication bias cannot be avoided because of the number of studies included. More trials with larger samples and good designs will be needed in the future to further evaluate the efficacy of MSC treatment in the management of HBV-associated ACLF patients.
In total, our pooled results suggest that MSC treatment could significantly reduce the mortality rate for Chinese HBV-ACLF patients compared with SMT. Also, no severe adverse events occurred, except for an increased incidence of fever without the need for a medical intervention. Generally, the MSC treatment is convenient, safe, and effective in the management of HBV-ACLF patients.
Source: Wolters Kluwer Health, Inc
Link: https://pubmed.ncbi.nlm.nih.gov/29727380/