EIN Presswwire, November 20 , 2023
News provided by Stemcell Bio
– Biostar Stem Cell Research Institute has received approval from Japan’s Ministry of Health, Labor and Welfare to treat Parkinson’s Disease.
– The combination of intravenous and spinal cord cavity administration is expected to increase the effectiveness of the treatment. This therapy may also be effective for other incurable neurological diseases.
– Stem cells are cultured in special media produced by Nature Cell, an affiliate of Biostar Stem Cell Research Institute. This proprietary culture media contributes to the safety and effectiveness of the treatment.
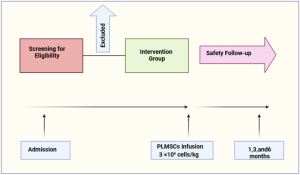
Korea’s leading adult stem cell research institute, Biostar Stem Cell Research Institute (Director, Dr. Jeongchan Ra), announced on the 20th that a regenerative medicine technology that treats Parkinson’s disease by administering autologous fat-derived stem cells cultured using patented technology into the intravenous and spinal cord cavity has been approved by the Japanese Ministry of Health and Welfare. The treatment will begin in December at the Shinjuku Clinic in Tokyo.
The approved stem cell treatment protocol involves administering 150 to 250 million adipose- tissue derived stem cells intravenously and 50 million cells into the spinal cord cavity five times at intervals of two to four weeks. The stem cells are either obtained from Biostar Stem Cell Research Institute in Korea or from JASC, a Japanese affiliate.
The approval of stem cell treatment for Parkinson’s disease marks a significant milestone in the clinical application of stem cells by Biostar Stem Cell Research Institute, which began its research in 2008. The institute has previously received regulatory approval for stem cell therapies for degenerative arthritis, severe lower extremity ischemia, and autoimmune diseases, administrated through intraarticular, intramuscular, and intravenous routes, respectively. The intravenous and spinal cord cavity administration of stem cells for Parkinson’s disease further demonstrates the versatility and safety of Biostar’s stem cell culture technology.
Stem cells vary greatly in safety and effectiveness depending on the culture method, highlighting the importance of rigorous quality management. Biostar Stem Cell Research Institute’s two-decade-long research on stem cell culture and treatment technologies, including the recently approved Parkinson’s disease therapy, holds promise for developing new avenues for treating neurological disorders. The specialized culture media developed by Biostar Stem Cell Research Institute, exclusively manufactured and supplied by its affiliate Nature Cell, plays a crucial role in enhancing the effectiveness and safety of these therapies.
Biostar Stem Cell Research Institute is committed to expanding treatment-approved hospitals across Japan and intensifying global outreach, aiming to make Japan a destination for Parkinson’s disease patients worldwide to regain their health.
Furthermore, Parkinson’s disease affects an estimated 10 million people worldwide, with a rapidly growing prevalence, and remains an incurable condition without a definitive treatment.
Source: EIN Presswwire